Toxicological data and environmental behavior of pyrene
Introduction
Pyrene is an organic compound with chemical formula C16H10, light yellow monoclinic crystal (pure product is colorless), aromatic, combustible[1], insoluble in water and soluble in ethanol and ether. It can carry out electrophilic substitution, such as halogenation, nitration, sulfonation and other reactions. Pyrene mainly exists in the distillate of coal tar pitch. Pyrene is an organic synthetic raw material, which can be oxidized to produce 1,4,5,8-naphthalene tetracarboxylic acid, which is used in dyes, synthetic resins, disperse dyes and engineering plastics; After acylation, the vat dye brilliant orange GR and other dyes can be prepared. It can also make pesticides, plasticizers, etc. On October 27, 2017, the list of carcinogens published by the international agency for research on cancer of the World Health Organization was preliminarily sorted out for reference. Pyrene was included in the list of three types of carcinogens.

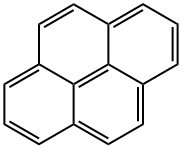
Picture 1 The source of Pyrene
It is combustible and can carry out electrophilic substitution, such as halogenation, nitration, sulfonation and other reactions, usually at position 3. It can be oxidized to pyrene quinone. Controlling the amount of oxidant can make pyrene oxidized to produce different carbonyl numbers. It has strong fluorescence, and the quantum yield can reach 0.99 in acetone. Pyrene crystal can emit light under voltage, which was originally used in electroluminescence research. It can be polymerized to obtain conductive polypyrene. This product is toxic. In case of acute poisoning, it first causes excitement, and then turns to inhibition, spasm, quadriplegia, irritation of eyes and upper respiratory tract mucosa. The LD50 of rats was 0.17mg/l and that of mice was 500.8g/kg. Water hazard level 3 (German regulation) (self assessment through list) this substance is extremely hazardous to water. Even in small quantities, do not allow undiluted or large quantities of products to come into contact with groundwater, waterways or sewage systems. Even a very small amount of products seeping underground can pose a danger to drinking water. Do not discharge materials into the surrounding environment without government permission. Explosion hazard: This product is combustible. Flammable in case of open fire and high heat. It is decomposed by high heat and releases toxic gas. The combustion (decomposition) products are carbon monoxide, carbon dioxide and black smoke with unknown composition.
Synthetic method
1. The content in high temperature tar is about 1.2% ~ 1.8%, which is prepared by separation and purification. Using anthracene oil as raw material, vacuum distillation, extraction, recrystallization and purification. The residue of distilled anthracene oil above 360 ℃ is used as raw material, the pyrene fraction is cut by distillation, benzene is used as solvent, washed with concentrated sulfuric acid, the base and unsaturated compounds are removed, and then the pure product is recrystallized with solvent oil. It can also be extracted from tobacco. Pyrene mainly exists in the distillate of coal tar pitch. The medium temperature asphalt is distilled under reduced pressure, and a small amount of direct superheated steam is introduced into the distillation kettle to cut the narrow fraction of pyrene, and then recrystallized with the mixed solution of solvent oil and ethanol or the mixed solution of benzene and solvent oil to obtain industrial pyrene with a purity of 95%.
Purpose
It can be directly oxidized to pyrene quinone. Organic synthetic raw materials can be oxidized to produce 1,4,5,8-naphthalene tetracarboxylic acid, which is used in dyes, synthetic resins, disperse dyes and engineering plastics; After acylation, the vat dye brilliant orange GR and other dyes can be prepared. It can also make pesticides, plasticizers, etc. Pyrene can be oxidized to produce 1,4,5,8-naphthalene tetracarboxylic acid, which is used in the production of dyes, synthetic resins, pesticides and other products.
Toxicological data and environmental behavior
Toxicity: low toxicity. Acute toxicity: ld502750mg / kg (oral to rats); 800mg / kg (mice orally); Lc50170mg / m3 (inhaled by rats). Subacute and chronic toxicity: rats inhaled 3.6mg/m3 × In April, hemoglobin and red blood cells decreased, lymphocytes decreased, leukocytes increased, hepatic glycogen increased, and proteinuria was seen microscopically; People with chronic inhalation concentration of 3 ~ 5mg / m3 have headache, fatigue, poor sleep, easy excitement, loss of appetite, increase of leukocyte and rapid increase of ESR; The concentration of 0.1mg/m3 in the workshop after chronic inhalation has no adverse effect. Inhalation, ingestion and percutaneous absorption. Health hazards: no acute poisoning was reported. Long term exposure of 3 ~ 5mg / m3 shows headache, fatigue, poor sleep, easy excitement, loss of appetite, increase of leukocytes, increase of ESR, etc. Less than 0.1mg/m3, no adverse effects.
Laboratory monitoring method
High performance liquid chromatography "analysis of organic compounds in municipal and industrial wastewater" translated by Wang keou and gas chromatography "manual for experimental analysis and evaluation of solid waste" translated by China environmental monitoring station, etc
Precautions for operation
In case of fire, the fire source should be cut off immediately. Wear gas masks and chemical protective clothing. Collect and transport to the open area for incineration. Fire extinguishing methods: foam, carbon dioxide, dry powder, 1211 extinguishing agent and sand. Water can cause a large amount of leakage, such as collection and recycling or waste after harmless treatment. Special protection is not required during general operation, but it is recommended to wear gas masks under special circumstances. Safety mask can be used. Wear work clothes. Wear chemical resistant gloves if necessary. Shower and change clothes after work. Avoid long-term repeated contact. In case of skin contact, take off the contaminated clothes and rinse thoroughly with soapy water and clean water. In case of eye contact, open the upper and lower eyelids immediately and rinse with flowing water for 15 minutes. See a doctor. If inhaled, leave the site to a place with fresh air. When ingested accidentally, the person who takes it by mistake shall drink sufficient warm water, induce vomiting and seek medical treatment.
Reference
1 Figueira-Duarte T M, Mullen K. Pyrene-based materials for organic electronics[J]. Chemical reviews, 2011, 111(11): 7260-7314.
Related articles And Qustion
See also
Lastest Price from Pyrene manufacturers
US $0.00-0.00/kg2025-11-06
- CAS:
- 129-00-0
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $999.00-666.00/g2025-04-21
- CAS:
- 129-00-0
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 5000