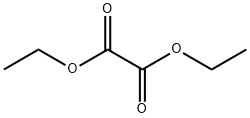
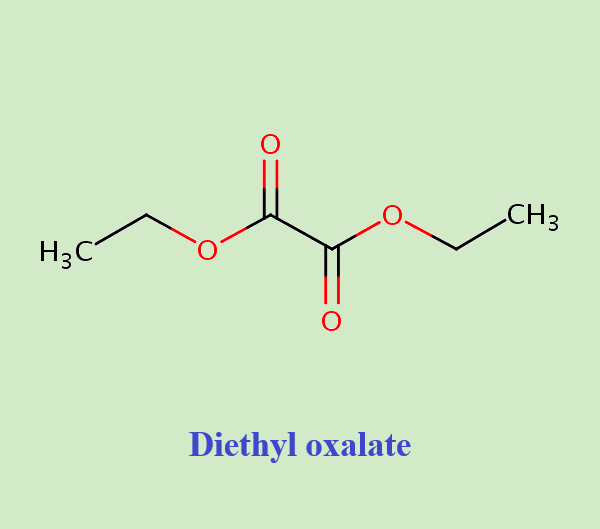
What are the uses of Diethyl oxalate?
Diethyl oxalate is a widely used organic ester compound with an important role in industrial manufacturing and chemical reactions. It can be used for following aspects:
(1) Reaction of organometallic reagents with ethyl trifluoroacetate or diethyl oxalate to obtain trifluoromethyl ketone or α-keto ester.
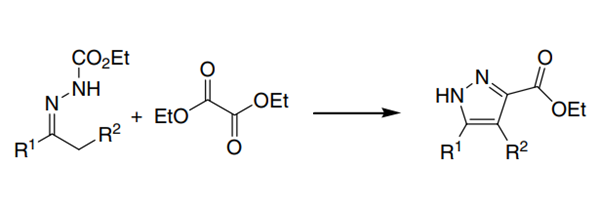
(2) The cyclisation of the dibasic ion of hydrazone with diethyl oxalate gives pyrazole-5-carboxylate.
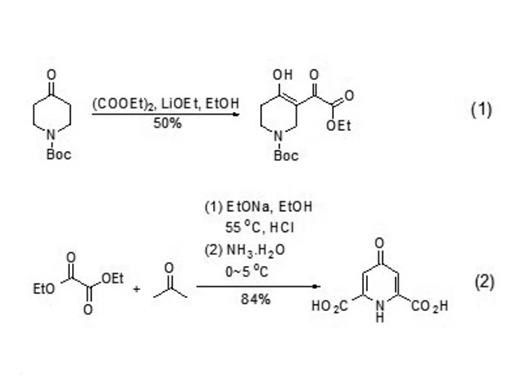
(3) The reaction of perfluorinated Grignard or lithium compounds with diethyl oxalate produces ketone esters and symmetrical or asymmetrical ketones.
(4) The reaction of bisguanidine compounds with diethyl oxalate produces (4,5-dioxo-2-imidazolidinyl) guanidine compounds. The reaction mechanism may be via a cyclic transition state.
(5) A Pd/α-Al2O3 nanocatalyst was synthesised as a catalyst for the CO oxidative coupling of diethyl oxalate with CeO2 as a promoter. Due to the incorporation of CeO2, the catalyst exhibited the highest activity and stability found to date, achieving extremely high CO conversion and diethyl oxalate selectivity.
(6) as a new potential protection product for decayed carbon substrates. Diethyl oxalate reacts with calcite powder to form an uncommon, new calcium oxalate phase for consolidating decaying carbonate matrices.
References:
[1] CREARY X. ChemInform Abstract: Reaction of Organometallic Reagents with Ethyl Trifluoroacetate and Diethyl Oxalate. Formation of Trifluoromethyl Ketones and α-Keto Esters via Stable Tetrahedral Adducts.[J]. ChemInform, 1988, 19 15. DOI:10.1002/chin.198815157.[2] TUAN THANH DANG Peter L Tung Thanh Dang. One-pot synthesis of pyrazole-5-carboxylates by cyclization of hydrazone 1,4-dianions with diethyl oxalate[J]. Tetrahedron Letters, 2007, 48 20: 3487-3624. DOI:10.1016/j.tetlet.2007.03.093.
[3] LOOMIS S. CHEN Christ T Grace J Chen. Fluoro-ketones VII. Synthesis of perfluoro mono- and di-ketones from perfluoro-grignard or lithium reagents and diethyl carbonate and diethyl oxalate[J]. Journal of Fluorine Chemistry, 1984, 26 3: 269-404. DOI:10.1016/S0022-1139(00)80935-5.
[4] S. HAYASHI. The Mechanism for the Reaction of Biguanides with Diethyl Oxalate[J]. Chemical & pharmaceutical bulletin, 1968, 16 1: 315-338. DOI:10.1248/CPB.16.471.
[5] JIN E, HE L, ZHANG Y, et al. A nanostructured CeO2 promoted Pd/α-alumina diethyl oxalate catalyst with high activity and stability?[J]. RSC Advances, 2014, 90: Page 48758 to 49546. DOI:10.1039/C4RA08170F.
[6] CLAUDIA CONTI . Diethyl oxalate as a new potential conservation product for decayed carbonatic substrates[J]. Journal of Cultural Heritage, 2014, 15 3: e3-e4. DOI:10.1016/j.culher.2013.08.002.
Related articles And Qustion
See also
Lastest Price from Diethyl oxalate manufacturers

US $10.00/kg2025-04-21
- CAS:
- 95-92-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $0.00/Kg/Drum2025-04-21
- CAS:
- 95-92-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 2000mt/year