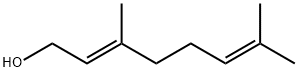
The synthesis method of Geraniol
Introduction
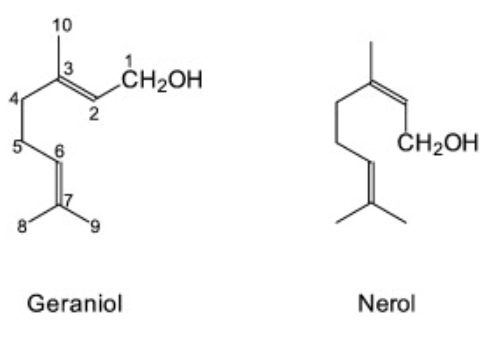
Geraniol (3,7-dimethylocta-trans-2,6-dien-1-ol) is an acyclic monoterpene alcohol with the chemical formula C10H18O. The product referred to as “geraniol” is a mixture of the two cis-trans isomers properly named geraniol (trans) and nerol (cis). Geraniol, a yellow oily liquid, has a gentleness and sweet rose breath for colourlessness. Geraniol is a rose with a host of essences; it is indispensable in blending raw material in various floral compounds, is widely used in floral type daily essences, and can also be used for the food flavour of odour types such as the fruit flavour types such as apple, strawberry, Chinese cassia tree, ginger, also can be used for making ester perfume[1].
Occurrence
Geraniol was isolated from Palmarosa oil, while nerol was obtained from the oil of neroli. It is a common constituent of several essential oils and occurs in Monarda fistulosa (> 95%), ninde oil (66.0%), rose oil (44.4%), palmarosa oil (53.5%) and citronella oil (24.8%). Geraniol is a clear to pale-yellow oil that is insoluble in water but in most organic solvents. It is emitted from the flowers of many species and is present in the vegetative tissues of many herbs. It often co-exists with geranial and neral, which are the oxidation products of geraniol. Pharate females (Tetranychus urticae) also emit geraniol.
Biological synthesis
Geraniol is known to be derived from geranyl diphosphate (GPP) by related synthases based on a common ionization-dependent reaction mechanism. GPP is synthesized via head-to-tail condensation of isopentenyl diphosphate (IPP) with dimethylallyl diphosphate (DMAPP). IPP is, in turn, synthesized from cytoplasmic acetate-mevalonate or the recently discovered plastidic non-mevalonate (pyruvate/triose-phosphate) pathway. In general, geraniol biosynthesis is via the mevalonate route, but in some plants, geraniol is known to be synthesized via the non-mevalonate pathway. Iijima et al. (2004) first purified and characterized geraniol synthase (GES) from the peltate glands of sweet basil. This geraniol synthase was highly specific and produced only geraniol. Subsequently, Yang et al. reported the isolation of a cDNA clone functionally expressed in Escherichia coli and identified it as a geraniol synthase from Cinnamomum tenuipilum.
Synthesis method
A preparation method of geraniol comprises the steps of adding linalool, tetrabutyl titanate and a vanadium derivative catalyst to a reaction kettle, heating the materials to 160-180 DEG C, stirring the materials for 10-16 hours and then carrying out hydrolysis, rectification at reduced pressure and discharging. The preparation method has the advantages of a simple process, small auxiliary material utilization amount, low cost, and reactions that are easy to control. Specifically, by the phantol of 0.8 weight part~1.0 weight part, the tetrabutyl titanate of 0.7 weight part~0.9 weight part, the vanadium derivative catalyst of 0.008 weight part~0.02 weight part adds reactor, be warming up to 160 ℃~180 ℃, stir 10 hours~16 hours, then through hydrolysis, rectification under vacuum, discharging[2].
References:
[1] AYUSHI AGRAWAL M B Zhiliang Yang. Engineering Yarrowia lipolytica for the biosynthesis of geraniol[J]. Metabolic Engineering Communications, 2023, 17. DOI:10.1016/j.mec.2023.e00228.[2] W. CHEN A M V. Geraniol — A review of a commercially important fragrance material[C]//76 4. 2010: 607-812. DOI:10.1016/j.sajb.2010.05.008.
You may like
Related articles And Qustion
See also
Lastest Price from Geraniol manufacturers

US $1.00/g2025-08-20
- CAS:
- 106-24-1
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 1000kg

US $0.00/KG2025-04-21
- CAS:
- 106-24-1
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month