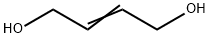The synthesis method of 2-Butene-1,4-diol
Description
2-Butene-1,4-diol (BED) is an organic compound with the chemical formula C4H8O2. This compound is a yellow oil that is soluble in Chloroform. As an important chemical intermediate, it is not only widely used for producing endosulfan, vitamins A and B6, but also used as a plasticizer of alkyd resin, fungicide of synthetic resin, and crosslinking agent. Consequently, it is of great value to develop an effective method to synthesize 2-Butene-1,4-diol.

Background
Among all of the synthetic methods for 2-butene-1,4-diol, selective hydrogenation of 2-butyne-1,4-diol is an effective strategy. Currently, the broadest commercial production method of 2-butene-1,4-diol is the selective hydrogenation of 2-butyne-1,4-diol (BYD) by nickel or palladium catalysts. However, the industrial nickel-based catalysis inclines to undergo full hydrogenation of BYD to butane-1,4-diol (BDO) and normally requires harsh conditions (15–30 MPa at up to 160 °C).
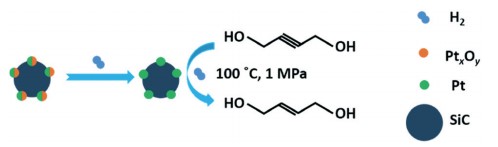
Silicon carbide supported platinum catalyst
Due to the selective hydrogenation of BYD by platinum-based catalysts under mild conditions (<150°C and <3 MPa), the selectivity of the relevant samples is low (about 80%). Furthermore, previous results reported that multiple side products, including γ-hydroxy butyraldehyde, n-butyraldehyde, n-butanol, crotyl alcohol, acetal, 2-isopropoxy-tetrahydrofuran, and butane, would be generated during the hydrogenation of BYD to BED in practical production. Hence, Shu et al prepared a series of new silicon carbide-supported platinum catalysts via a simple approach of incipient wetness impregnation. Specifically, the Pt/SiC catalyst with a low loading amount of Pt (0.5 wt%) exhibited an outstanding selectivity of 2-Butene-1,4-diol (up to 96%) with excellent conversion (up to 96%) under 100 °C and p(H2) = 1 MPa after 10 h reaction. The side product was only BDO, which may be beneficial to the reaction operation and the sequential separation process.
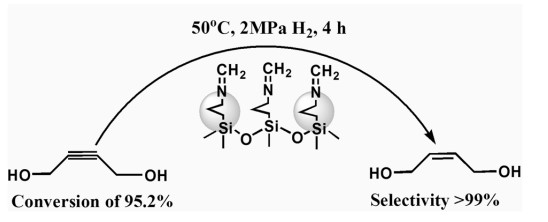
Schiff Base Modified Pd Catalyst
In order to achieve an efficient synthesis of BED, Li et al report a Pd nanocatalyst modified by Schiff base, which shows an excellent catalytic performance in selective hydrogenation of 2-butyne-1,4-diol to 2-Butene-1,4-diol. The conversion of 2-butyne-1,4-diol was as high as 95.2% with ca. 100% BED selectivity under the mild reaction conditions (50 °C, 2 MPa H2, and 4 h) and additive-free. The above results are much better than that of commercial Lindlar catalyst, and the improved catalytic performance is attributed to the strong metal–support interaction derived from the coordination of nitrogen sites (Schiff-base) to Pd NPs, based on catalyst characterization results.
References
[1] Shu M, et al. Efficient selective hydrogenation of 2-butyne-1,4-diol to 2-butene-1,4-diol by silicon carbide supported platinum catalyst. Catalysis Science & Technology, 2019; 10: 327-331.
[2] Li H, et al. A Schiff Base Modified Pd Catalyst for Selective Hydrogenation of 2-Butyne-1,4-diol to 2-Butene-1,4-diol. Catalysis Letters, 2020; 150: 2150–2157.
Related articles And Qustion
See also
Lastest Price from 2-Butene-1,4-diol manufacturers

US $45.00-40.00/kg2025-07-18
- CAS:
- 110-64-5
- Min. Order:
- 250kg
- Purity:
- ≥99.0% (GC)
- Supply Ability:
- 50 tons

US $10.00/ASSAYS2025-05-04
- CAS:
- 110-64-5
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 10 ton