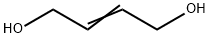Exploring the Versatility and Safety of 2-Butene-1,4-diol in Industrial Chemistry
2-Butene-1,4-diol is a colorless viscous liquid, it is used in chemical synthesis.

Use
2-Butene-1,4-diol has been used as a cross-linking agent for various polymers and is also able to form hydroxyl groups through allylation reactions.
Synthesis
In one industrial chemical synthesis, acetylene reacts with two equivalents of formaldehyde to form butyne-1,4-diol. Hydrogenation of butyne-1,4-diol gives 2-Butene-1,4-diol. It is also made on an industrial scale from maleic anhydride in the Davy process, which is first converted to the methyl maleate ester, then hydrogenated. Other routes are from butadiene, allyl acetate and succinic acid.
Reactivity
2-Butene-1,4-diol forms furan (narcotic)when treated with dichromate in acidic solution.Dehydration of the cis-isomer overacid catalysts yields 2,5-dihydrofuran (narcotic). Halogens form substitution or additionproducts, 4-halobutenols, or 2,3-dihalo-1,4-butanediol. These are toxic compounds.
Safety
2-Butene-1,4-diol is a depressant of the Centralnervous system. Inhalation toxicity isvery low due to its low vapor pressure. Theoral LD50 value in rats and guinea pigs is1.25 mL/kg. It is a primary skin irritant.
You may like
Related articles And Qustion
Lastest Price from 2-Butene-1,4-diol manufacturers

US $45.00-40.00/kg2025-07-18
- CAS:
- 110-64-5
- Min. Order:
- 250kg
- Purity:
- ≥99.0% (GC)
- Supply Ability:
- 50 tons

US $10.00/ASSAYS2025-05-04
- CAS:
- 110-64-5
- Min. Order:
- 1ASSAYS
- Purity:
- 99%
- Supply Ability:
- 10 ton