The synthesis and metabolism of
Introduction
Taurine is a sulfur-containing amino acid with simple structure in animals. Its chemical name is 2-aminoethanesulfonic acid[1]. Its molecular formula is C2H7NO3S and its molecular weight is 125.15. It is odorless and slightly sour. Its dilute solution is neutral and stable to heat. It combines with cholic acid in human and animal bile and exists in the form of binding; In brain, ovary, heart, liver, milk, pineal gland, pituitary, retina, adrenal gland and other tissues, it exists in free form, with a total amount of 12-18g, but does not participate in protein synthesis. Taurine is a conditionally essential amino acid for human body and plays an important role in the development of fetal and infant nervous system. Taurine can be widely used in medicine, food additives, fluorescent brighteners, organic synthesis and other fields. It can also be used as biochemical reagent, wetting agent, pH buffer and so on.

Picture 1 Taurine powders
Performance
Taurine is an amino acid transformed from sulfur-containing amino acids, also known as taurine, taurine, taurine choline and taurine[2]. Taurine is widely distributed in various tissues and organs in the body, and mainly exists in interstitial fluid and intracellular fluid in free state. It was first found in bull bile and got its name, but it has long been considered as a non functional metabolite of sulfur-containing amino acids. Taurine is a sulfur-containing amino acid in animals, but it is not a component of protein. Taurine is widely distributed in human and animal brain, heart, liver, kidney, ovary, uterus, skeletal muscle, blood, saliva and milk in the form of free amino acids, with the highest concentration in pineal gland, retina, pituitary gland, adrenal gland and other tissues. In mammalian heart, free taurine accounts for 50% of the total free amino acids.
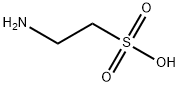
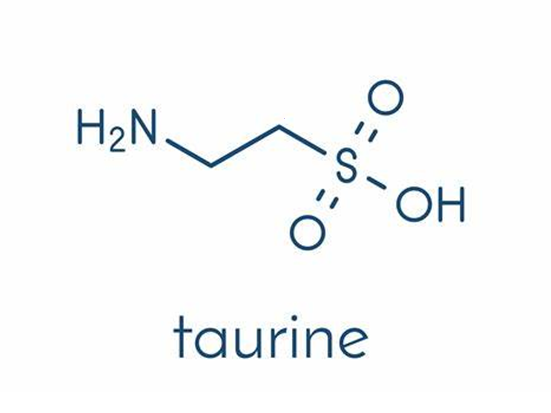
The chemical structure formula of taurine is H2N-CH2-CH2-SO3H, and the chemical name is β- Aminoethanesulfonic acid or 2-aminoethanesulfonic acid, with relative molecular weight of 125.15, monoclinic prismatic rod-shaped white crystal, melting point of 305.11 ℃, non-toxic, odorless, slightly acidic and stable to heat. Taurine is soluble in water, with a solubility of 0.5% at 12 ℃, pH of its aqueous solution of 4.1 ~ 5.6, solubility of 0.004% at 17 ℃ in 95% ethanol, and insoluble in absolute ethanol, ether and acetone. Taurine is an organic osmotic regulator. It not only participates in the regulation of cell volume, but also provides the basis for the formation of bile salts. It also plays an important role in the modulation of intracellular free calcium concentration. Although taurine is a special amino acid not included in proteins, taurine is the most abundant amino acid in brain, retina and muscle tissue. Taurine is widely used, such as in the function of central nervous system, cell protection, cardiomyopathy, renal insufficiency, abnormal development of renal function and retinal nerve injury. Almost all eye tissues contain taurine. The quantitative analysis of rat eye tissue extract showed that taurine was the most abundant amino acid in retina, vitreous, lens, cornea, iris and ciliary body. Many studies have found that taurine is an active substance that regulates the normal physiological activities of the body. It has the functions of anti-inflammatory, analgesic, maintaining the osmotic pressure balance of the body, maintaining normal visual function, regulating the calcium balance of cells, reducing blood sugar, regulating nerve conduction, participating in endocrine activities, regulating lipid digestion and absorption, increasing the contractility of the heart, improving the immune capacity of the body, and enhancing the antioxidant capacity of cell membrane Protect a wide range of biological functions such as cardiomyocytes.
Synthesis and metabolism
In addition to the intake of taurine directly from the diet, the animal body can also biosynthesis in the liver. The intermediate product of methionine and cysteine metabolism, cysteine, is decarboxylated to taurine by cysteine decarboxylase (CSAD), and then oxidized to taurine. CSAD is considered to be the rate limiting enzyme of taurine biosynthesis in mammals, and compared with other mammals, the activity of human CSAD is lower, which may be due to the low taurine synthesis ability in human body. Taurine can participate in the formation of taurocholic acid and hydroxyethyl sulfonic acid after decomposition in vivo. The amount of taurine required depends on cholic acid binding capacity and muscle content.
In addition, taurine is excreted in free form through urine or in the form of cholate through bile. The kidney is not only the main organ for excreting taurine, but also an important organ for regulating the content of taurine in the body. When taurine is excessive, the excess is excreted with urine; When taurine is insufficient, the kidney reduces taurine excretion through reabsorption. In addition, a small amount of taurine is discharged through the intestine.
Reference
1 Jiang Yimin, Zhang Shuhua, Xu Qing, Xiao Yu Synthesis, crystal structure and bioactivity of binuclear Cu (Ⅱ) - taurine glycidyl salicylaldehyde Schiff base complex two thousand and three. 2003
2 Gu Yanlong, Shi Feng, Deng Youquan Separation of solid mixture of taurine and sodium sulfate by room temperature ionic liquid extraction Journal of chemistry, 2004
Related articles And Qustion
See also
Lastest Price from Taurine manufacturers

US $300.00-13.00/KG2025-04-29
- CAS:
- 107-35-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20000KG

US $100.00/KG2025-04-21
- CAS:
- 107-35-7
- Min. Order:
- 1KG
- Purity:
- 99%min
- Supply Ability:
- 200TON