Reaction of artemisinin with other substances
Introduction
Artemisinin is an organic compound with molecular formula of C15H22O5 and relative molecular weight of 282.34. Artemisinin is a colorless acicular crystal with a melting point of 156 ~ 157 ℃. It is easily soluble in chloroform, acetone, ethyl acetate and benzene, soluble in ethanol and ether, slightly soluble in cold petroleum ether and almost insoluble in water. Because of its special peroxy group, it is unstable to heat and is easy to decompose under the influence of moisture, heat and reducing substances[1].

Picture 1 Artemisinin plant
Artemisinin is the best drug to treat drug resistance of malaria. The combination therapy based on artemisinin drugs is also the most effective and important means to treat malaria at present. But in recent years, with the development of research, more and more artemisinin's other functions have been discovered and applied, such as anti-tumor, treatment of pulmonary hypertension, anti diabetes, embryotoxicity, antifungal, immunomodulation, antiviral, anti-inflammatory, anti pulmonary fibrosis, antibacterial, cardiovascular effects and other pharmacological effects. In October 2015, Tu youyou and two other scientists won the 2015 Nobel Prize in physiology or medicine for their contributions to the creation of new antimalarial drugs artemisinin and dihydroartemisinin.
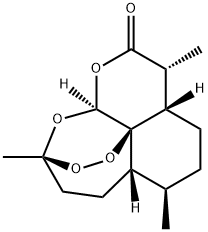
The molecular formula of artemisinin is c15h22o5 and the molecular weight is 282.34. It is a new sesquiterpene lactone with peroxy bond and δ- Lactone ring has a 1,2,4-trioxane structural unit including peroxide, which is very rare in nature. Its molecule includes seven chiral centers. Its biogenic relationship belongs to amorphane type, which is characterized by the CIS connection of a and b rings, the trans relationship between isopropyl and bridgehead hydrogen, and the carbon frame of a ring in artemisinin is interrupted by an oxygen atom. Artemisinin is a colorless acicular crystal with a melting point of 156 ~ 157 ℃ (C = 1.64 chloroform). It is easily soluble in chloroform, acetone, ethyl acetate and benzene, soluble in ethanol and ether, slightly soluble in cold petroleum ether and almost insoluble in water. Because of its special peroxy group, it is unstable to heat and is easy to decompose under the influence of moisture, heat and reducing substances.
Peroxy group reaction
The reaction between artemisinin and triphenylphosphorus can prove that artemisinin contains an equivalent peroxy group. The method is to reflux artemisinin in triphenylphosphorus and xylene solution with nitrogen, add formaldehyde and water, stir, wash the organic layer with water, combine the water layer and acidic solution, add alkali, extract with peroxide free ether, dry with anhydrous sodium sulfate, remove ether, and measure the weight of triphenylphosphorus, The results showed that the molar number of triphenylphosphorus consumed was similar to that of artemisinin.
Color reaction
Color reaction is a simple and feasible method to identify artemisinin. There are many reports, mainly including the following: (1) condensation reaction of p-Dimethylaminobenzaldehyde: take about 10mg of the experimental product artemisinin, dissolve it with 2ml of ethanol, add 1ml of p-Dimethylaminobenzaldehyde reagent, heat it in a water bath, and the solution shows blue purple reaction. (2) Hydroxamic acid iron reaction: take about 10mg of the experimental product artemisinin, dissolve it in 1ml methanol and add it φ= 4 ~ 5 drops of 7% hydroxylamine hydrochloride methanol solution, heat it on the water bath to boiling, cool it, add dilute hydrochloric acid to adjust it to acidic, and then add it φ= 1% FeCl3, 1 ~ 2 drops of ethanol solution, and the solution reacts purple red. (3) 2,4-dinitrophenylhydrazine reaction: take about 10mg of the experimental product artemisinin, dissolve it in 1ml chloroform, drop it on the filter paper, spray it with 2,4-dinitrophenylhydrazine test solution, and bake it in an oven at 80 ℃ for 10min to produce yellow spots. (4) Basic m-dinitrobenzene reaction: take about 10mg of the experimental product artemisinin, dissolve it in 2ml ethanol and add it φ= The ethanol solution of 2% m-dinitrobenzene and saturated KOH ethanol solution are several drops respectively. The water bath is slightly hot, and the solution reacts red
Reaction with alkali
Artemisinin is dissolved in methanol, and potassium carbonate is dissolved in water. The potassium carbonate solution is slowly added into the artemisinin methanol solution under stirring to make it evenly mixed into a clear solution. Keep the temperature at 20 ~ 22 ℃ for 1h, add water, extract twice with ether, wash the ether layer twice with a small amount of water, and use water for the water layer φ= Acidified with 10% hydrochloric acid to pH = 2, and then extracted with ether for 3 times. The ether layer is washed to neutral with water and dried with anhydrous sodium sulfate for 2 ~ 3H. The ether layer is pumped dry under reduced pressure. The residue is put into the refrigerator for liquid filtration. There are semi-solid precipitates. Add a little methanol, cool, precipitate acicular crystals, which are compounds. Filter and recrystallize once to obtain more refined crystals.
Reaction with acid
Artemisinin is added into the mixture of glacial acetic acid and concentrated H2SO4, shaken and dissolved. The solution is light brownish yellow and slightly fluorescent after being placed at 25 ℃ for 16 ~ 17h. The reaction solution is poured into equal volume of ice water, stirred and extracted with chloroform for 3 times. The chloroform layer is washed to neutral with water, dried with anhydrous sodium sulfate, and the organic solvent is removed under reduced pressure to obtain coarse crystallization and recrystallization for 2 times, so as to obtain the flake crystallization of compound VII (VII in Figure 1), with melting point of 144 ~ 146 ℃, (C =. 2.1 chloroform).
Reference
1 Wang Zongde, sun Fanghua Research Progress on physical and chemical properties and determination methods of artemisinin [J] Journal of Jiangxi Agricultural University, 1999 (04): 606-611
Related articles And Qustion
Lastest Price from Artemisinin manufacturers

US $1200.00-1100.00/ton2025-10-01
- CAS:
- 63968-64-9
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $5.00-0.50/KG2025-05-08
- CAS:
- 63968-64-9
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS