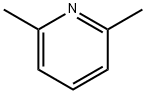
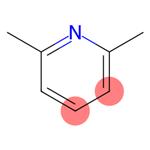
The structure of 2,6-Lutidine
Description
2,6-Lutidine (2,6-dimethylpyridine, C7H9N),(I), is present in many complexes coordinated to a metal center. The free base (unprotonated and not coordinated to any metal center) has also been observed in the crystal structures of three solvates, usually hydrogen bonded to the principal component of the structure. However, the crystal structure of 2,6-Lutidine itself has not been reported to date, probably due to the differences associated with obtaining suitable single crystals of 2,6-Lutidine, which is liquid at room temperature.
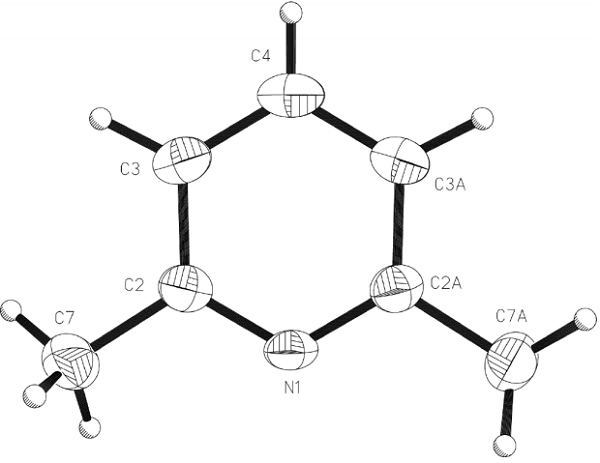
Structure
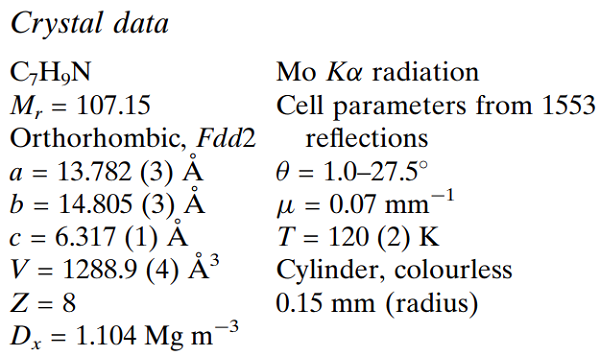
In the non-centrosymmetric space group Fdd2, the asymmetric unit comprises half a molecule of (I), with a crystallographic diad axis passing through atoms N1 and C4. Molecules of (I) are linked into one-dimensional chains propagating along the diad axis (parallel to [001]) by linear C-H.....N interactions (H4.....N1i = 2.63 A and C4-H4....N1 = 180°; symmetry code: (i) x, y, 1+ z). Adjacent chains are parallel such that the structure is macroscopically polar; this observation may interest researchers seeking organic molecular materials for non-linear optic (NLO) applications, mainly frequency doubling through second harmonic generation (SHG). The presence of the methyl substituents in the 2- and 6-positions prevents the adoption of an edge-to-face geometry between molecules (that might otherwise be expected), and the planes through the pyridyl rings of molecules in adjacent chains remain essentially parallel[1].
Uses
Synthesis of 2,6-divinylpyridine (2,6-DVP) and 2-methyl-6-vinylpyridine (2M6VP) was achieved by side-chain alkylation of 2,6-lutidine using formaldehyde (37 wt/v) as an alkylating agent in heterogeneous conditions at atmospheric pressure and at a reaction temperature of 300 C over alkali and alkaline metal ion modified zeolites. A mixture of 2,6- divinylpyridine and 2-methyl,6-vinylpyridine was formed by the alkylation of the 2,6-lutidine over Li, Na, K, Rb, Cs, Mg, Ca, Sr, and Ba metal ion modified zeolites[2].
References
[1] John E. Davies, Anthony J. Kirby, Andrew D. Bond. “2,6-Lutidine.” Acta crystallographica. Section E, Structure reports online 57 12 (2004): o1242–o1244.
[2] G. Madhavi, K. V. Raghavan, S. J. Kulkarni. “Side-chain alkylation of 2,6-lutidine to 2,6-divinylpyridine over basic zeolites.” Journal of Porous Materials 14 4 (2007): 379–385.
You may like
Related articles And Qustion
See also
Lastest Price from 2,6-Lutidine manufacturers

US $10.00/KG2025-04-21
- CAS:
- 108-48-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt
US $0.00/kg2025-04-15
- CAS:
- 108-48-5
- Min. Order:
- 20kg
- Purity:
- 99%
- Supply Ability:
- 20 tons