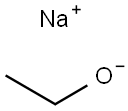
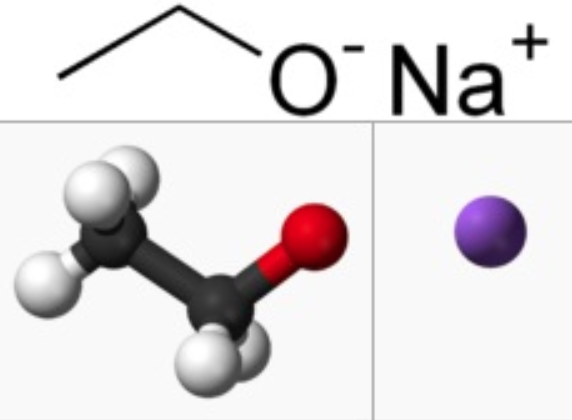
The Preparation of Sodium Ethoxide
Sodium ethoxide (also is the organic compound with the formula C2H5ONa) is a white to yellowish powder that dissolves in polar solvents such as ethanol. It is commonly used as a strong base [1]. Sodium Ethoxide is generally immediately available in most volumes. High purity, submicron and nanopowder forms may be considered. American Elements produces to many standard grades when applicable, including Mil Spec (military grade); ACS, Reagent and Technical Grade; Food, Agricultural and Pharmaceutical Grade; Optical Grade, USP and EP/BP (European Pharmacopoeia/British Pharmacopoeia) and follows applicable ASTM testing standards. Typical and custom packaging is available. Additional technical, research and safety (MSDS) information is available as is a Reference Calculator for converting relevant units of measurement [2].
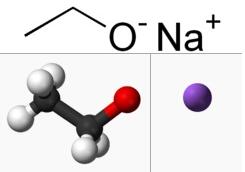
Sodium ethoxide can be prepared by adding sodium metal to anhydrous ethanol. Cooling the solution will cause the compound the crystallize. Do this reaction in a dry atmosphere, to limit hydrolysis [3]. Few procedures have been reported to the anhydrous solid. Instead the material is typically prepared in a solution with ethanol. It is commercially available and as a solution in ethanol. It is easily prepared in the laboratory by treating sodium metal with absolute ethanol:
2 C2H5OH + 2 Na → 2 C2H5ONa + H2
An alternative, cheaper route involves the reaction of sodium hydroxide with anhydrous ethanol. This reaction suffers from incomplete conversion to the alkoxide, but for less stringent applications, full conversion is unimportant. The salt product may be purified by precipitation in a solution of anhydrous acetone. An improvement to this process involves removing generated water with molecular sieves [1].
In addition, sodium ethoxide is a strong base, and is therefore corrosive. The solid gradually turns dark on storage in dry air because of oxidation. In moist air, it hydrolyzes rapidly to sodium hydroxide. The conversion is not obvious and typical samples of NaOEt are contaminated with NaOH [1]. Sodium ethoxide should be stored in air-tight containers, or in a desiccator. Schlenk flasks are a good choice [3].
Reference
[1] http://wikipedia.moesalih.com/Sodium_ethoxide
[2] https://www.americanelements.com/sodium-ethoxide-141-52-6
[3] http://www.sciencemadness.org/smwiki/index.php/Sodium_ethoxide
Related articles And Qustion
See also
Lastest Price from Sodium ethoxide manufacturers

US $10.00/kg2025-04-21
- CAS:
- 141-52-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100

US $0.00-0.00/Kg2025-04-21
- CAS:
- 141-52-6
- Min. Order:
- 1Kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons