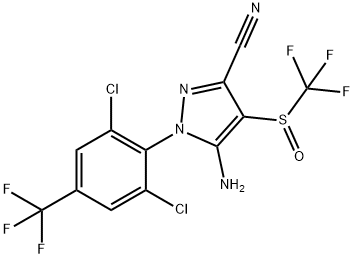
Fipronil: Clinical applications, Mechanism, Toxicity
Fipronil is a broad-spectrum insecticide that belongs to the phenylpyrazolechemical family. Fipronil is a phenylpyrazole compound and was developed as a useful insecticide in the mid-1990s. It is effective against some insects such as the Colorado potato beetle and certain cotton pests that have become resistant to the existing insecticides. Fipronil is much more toxic to insects than to mammals, another advantage it has as an insecticide [1].
Clinical applications
Fipronil was discovered in 1987 and was developed initially for use in pest control in agriculture and public health. In dogs and cats,fipronil is available as a high-volume spray or a low-volume spot-on, with activity against ticks, fleas and ear mites. Hair shed from dogs for up to 2 weeks after topical treatment retains sufficient fipronil to kill dust mites (Dermatophagoides spp) coming in contact. There is also some evidence that the speed of kill of ticks may be sufficient to reduce or prevent the transmission of a number of disease agents, including Ehrlichia canis, Borrelia burgdorferi and Anaplasma phagocytophilum.
Mechanism of action
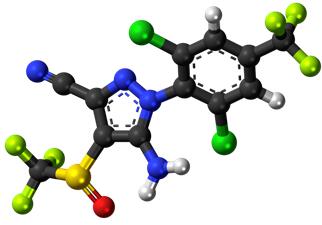
Recent investigations suggest that the mechanism of action of fipronil is complex, involving multiple interactions of both parent fipronil and its oxidation product, fipronil sulfone, on GABA-gated and glutamate-gated chloride channels in the insect nervous system. Both fipronil and fipronil sulfone inhibit GABA receptors as well as desensitizing and nondesensitizing GluCls, though the activity of fipronil sulfone is much higher than fipronil for desensitizing GluCls. The net result of insect exposure to fipronil is blockade of inhibitory nerve transmission, resulting in hyperexcitability and death of susceptible parasites. GluCls have been observed only in invertebrates The binding affinities of fipronil and fipronil sulfone to mammalian GABAA receptors are much less than in arthropods(GABAA receptor binding IC50 human:insect of 135 and 17 respectively) with no binding to other types of mammalian GABAreceptor, accounting (in combination with the low systemic bioavailability after dermal administration) for the selectivity of action. However, fipronil and its metabolites and degradation products are highly toxic to some species of fish.
Pharmacokinetics
Fipronil is not thought to be significantly absorbed from topical sites of application but to translocate dermally, being confined to the lipids of hair follicles and sebaceous glands. From this reservoir, drug is released for many weeks, accounting for the sustained activity against fleas and ticks. On the basis of studies in humans, inadvertently ingested fipronil appears to be rapidly and well absorbed from the gastrointestinal tract, extensively metabolized to the sulfone and subject to significant enterohepatic recirculation. The elimination half-life is 7–8 h for fipronil but 7–8 d for fipronil sulfone [2-3].
Reference
[1] Narahashi, T. (2010). Chapter 31 - Neurophysiological Effects of Insecticides. Hayes' Handbook of Pesticide Toxicology (Third Edition). R. Krieger. New York, Academic Press: 799-817.
[2] Page, S. W. (2008). Chapter 10 - Antiparasitic drugs. Small Animal Clinical Pharmacology (Second Edition). J. E. Maddison, S. W. Page and D. B. Church. Edinburgh, W.B. Saunders: 198-260.
[3] https://www.sciencedirect.com/topics/neuroscience/fiproni
You may like
Related articles And Qustion
See also
Lastest Price from Fipronil manufacturers
US $0.00-0.00/KG2025-11-29
- CAS:
- 120068-37-3
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $0.00/kg2025-09-28
- CAS:
- 120068-37-3
- Min. Order:
- 100kg
- Purity:
- 5
- Supply Ability:
- 10000