The preparation of pinacol
Introduction
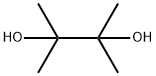
Pinacol , tetramethylethylene glycol, is also known as 2,3-dimethyl-2,3-dihydroxybutane(picture 1)[1]. Precipitated from ethanol or ether is a colorless needle crystal, and from water is a quadrilateral flake crystal hexahydrate. The relative molecular mass is 118.15. The relative density is 0.967 (hexahydrate, 15℃). Melting point 43 ° C (anhydrous), 47 ° C (hexahydrate). Pinacol (Pinacol), white or light yellow crystal, is an important intermediate for the preparation of olefins and ketones, widely used in rubber, pesticides, pharmaceutical and other fine chemical products synthesis.

Picture 1 The physical picture of pinacol
Preparation of pinacol by acetone method
At present, the domestic production process of synthetic pinacol mainly adopts the reductive coupling of aldehydes, ketones and carbonyl compounds[1-3]. Secondary polymerization to obtain pinacol, and then filtration, cooling and crystallization to obtain water-containing pinacol. The reaction conditions of the acetone method are harsh, the reaction is violent, the selectivity is poor, many by-products are produced, the yield is usually lower than 50%, and the production cost is high. In order to obtain high yield, it is necessary to increase the reaction rate, and at the same time to ensure that acetone does not volatilize, which is extremely unsafe in industrial operation, prone to explosion, and the heavy metal toxicity is serious, causing serious environmental pollution. For this reason, some manufacturers use tetramethylethylene and hydrogen peroxide as raw materials, platinum-palladium composite metal as catalyst, zeolite molecular sieve as carrier, and obtain high-purity pinacol products through hydroxyl addition reaction. The process has the characteristics of high yield (over 90%), non-toxic raw materials and catalysts, no three-waste emissions in the production process, and low cost. It is environmentally friendly, conducive to the sustainable development of the industry, and has broad market application prospects.
Reductive coupling of carbonyl compounds to prepare pinacol
Pinacol (vicinal diol) is an important intermediate for the preparation of alkenes and ketones, and is widely used in the synthesis of fine chemical products such as pesticides and medicines. One of the most effective synthetic methods for pinacol is the reductive coupling of carbonyl compounds , usually obtained by the interaction of carbonyl compounds with metal reagents or metal complexes, and generally follows a single electron transfer mechanism. In addition to bimolecular reduction products, there are sometimes single molecule reduction products in the reaction; the coupling product has two chiral centers. , which makes the efficient synthesis of pinacol more difficult. In order to effectively control the chemical selectivity of the reaction and the stereoselectivity of the product, the search for new metal reagents and new reaction systems has always been the focus of attention and research. Recently, some scholars Ultrasonic radiation is used to promote the reductive coupling reaction of metals acting on carbonyl compounds to prepare pinacol[1], but there are few reports on the synthesis of pinacol by microwave radiation and electrochemical methods. In 1986, Gedye et al. [2] carried out esterification, hydrolysis, oxidation and nucleophilic substitution reactions in a microwave oven, and Giguere et al. [3] carried out the Diels-Alder cycloaddition reaction of anthracene and dimethyl maleate. Research, as a high-frequency electromagnetic wave, microwave can promote many chemical reactions, with fast reaction speed, less side reactions, high yield, and easy synthesis of products.
Green oxidant hydrogen peroxide to synthesize pinacol by olefin oxidation
Pinacol is a good solvent and an important fine organic chemical intermediate for synthetic rubber, pesticides and new pharmaceutical anticancer drugs, and has a wide range of uses. At present, the domestic production process of pinacol mainly adopts the reductive coupling of aldehydes and ketones, that is, acetone is used as raw material, alkaline earth metal Mg, metal Al, IIIA metal indium (In), transition metals and their compounds and microwave solvent-free and other catalytic system conditions to synthesize pinacol. The acetone method has harsh reaction conditions, severe reaction, easy explosion, poor selectivity, many by-products, low yield (49%), high production cost (150,000 yuan/t), and heavy metal toxicity, serious environmental pollution. With the improvement of environmental protection awareness and the increasing promotion of the concept of green chemistry, the selection of green oxidant hydrogen peroxide to oxidize olefins to synthesize vicinal diols will have more and more development value. Tetramethylethylene and hydrogen peroxide are used as raw materials, platinum-palladium catalyst, and zeolite molecular sieve as carrier to synthesize high-purity pinacol. It has the characteristics of non-toxicity, no three wastes in the production process, and low cost. Compared with the acetone synthesis route, this route has higher yield, greatly reduces environmental pollution, is beneficial to the sustainable development of the industry, and has broad market application prospects.
Reference
1 Yang Zhenqiang, Wang Hui, Li Guoming, Li Rui, Qu Fengbo Synthesis and characterization of spiro [9h-fluorene-9,9 '- [9h] oxaanthracene] - 2-borate pinacol ester [J] Aging and application of synthetic materials, 2021,50 (06): 36-37
2 Zhu Yuan, Zhao Yazhi, Tian Chen, Chen Yueyuan, Ning Hongxin, Hou Wenbin, Li Yiliang Study on fluorine 19 labeling method of aryl boric acid pinacol ester [J] Modern medicine and clinic, 2021,36 (06): 1097-1103
3 Zhang Ye Study on series hemipinacol rearrangement based on the synthesis of furan and spiro [4.5] decane [D] Lanzhou University, 2020
Lastest Price from Pinacol manufacturers
US $0.00-0.00/KG2025-12-02
- CAS:
- 76-09-5
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $45.00/kg2025-04-21
- CAS:
- 76-09-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 tons