Pharmacological action of trimethoprim
Introduction
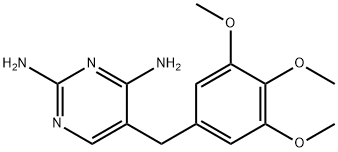
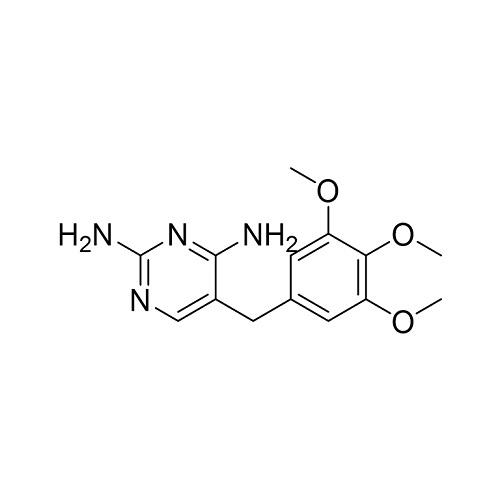
Trimethoprim, abbreviated as TMP, is an organic compound with chemical formula C14H18N4O3, white or to pale yellow crystalline powder, odorless, bitter in taste, slightly soluble in chloroform, slightly soluble in ethanol or acetone, almost soluble in water Insoluble, soluble in glacial acetic acid[1]. Trimethoprim is a synthetic broad-spectrum antibacterial agent, used alone for respiratory infections, urinary tract infections, intestinal infections and other diseases(picture 1), and can be used to treat sepsis, meningitis, otitis media, typhoid fever, shigellosis ( Bacillary dysentery) etc.
Picture 1 Trimethoprim tablets
Pharmacological action
Trimethoprim is a bacterial dihydrofolate reductase inhibitor and a sulfonamide synergist[2]. The principle of its antibacterial effect is to interfere with the folic acid metabolism of bacteria. It is mainly to selectively inhibit the activity of bacterial dihydrofolate reductase, so that dihydrofolate cannot be reduced to tetrahydrofolate, thereby inhibiting the growth and reproduction of bacteria. Trimethoprim has antibacterial activity against most gram-positive and gram-negative bacteria; in addition, trimethoprim also has a certain effect on Plasmodium and some fungi, such as Nocardia, histoplasma, and yeast. Among gram-positive bacteria, Streptococcus pneumoniae is sensitive to trimethoprim. Among gram-negative bacteria, Escherichia coli, Salmonella, Proteus mirabilis, Bacillus pneumoniae, Bacillus dysenteriae, Bacillus typhi, and Bacillus pertussis are sensitive to trimethoprim. Trimethoprim has no antibacterial effect on Pseudomonas aeruginosa, meningococcus and Alcaligenes.
Pharmacokinetics
Trimethoprim is completely absorbed after oral administration, and it can absorb more than 90% of the administered dose. After oral administration of 0.1 g, the peak plasma concentration of about 1 mg/mL is reached after 1 to 4 hours. The apparent volume of distribution of trimethoprim is 1.2 to 2.2 L/kg. After the drug is absorbed, it is widely distributed in tissues and body fluids, and the concentration in kidney, liver, spleen, lung, muscle, bronchial secretions, saliva, vaginal secretions, prostate tissue and prostatic fluid all exceeds the blood concentration. Trimethoprim can pass through the blood-cerebrospinal fluid barrier to the cerebrospinal fluid. When the meninges are not inflamed, the cerebrospinal fluid drug concentration is 30% to 50% of the blood drug concentration, and it can reach 50% to 100% when there is inflammation. Trimethoprim can also pass through the blood-placental barrier, and the concentration of the drug in the fetal blood circulation is similar to that in the maternal blood. The concentration of trimethoprim in milk is close to or higher than the blood concentration, and the drug concentration in the aqueous humor is about 1/3 of the blood concentration. The protein binding rate of trimethoprim is 30% to 46%, and the elimination half-life is about 8 to 10 hours, and the half-life of anuria can be extended to 20 to 50 hours. The drug is mainly excreted in the urine through glomerular filtration and renal tubular secretion. About 50% to 60% of the dose can be excreted in 24 hours, of which 80% to 90% is excreted in the form of the original drug, and the rest is excreted in the form of metabolites. The average urinary drug concentration is 90-100 mg/L, and the peak concentration in urine is about 200 mg/L. A small amount of the drug (about 4% of the administered dose) can be excreted in the bile and feces. Trimethoprim can be removed by hemodialysis, but not by peritoneal dialysis.
Interactions with other drugs
1. The combination of trimethoprim and sulfa drugs can double block the folic acid synthesis and metabolism of bacteria, have a synergistic antibacterial effect, and can turn its antibacterial effect into a bactericidal effect.
2. The combination of trimethoprim and berberine, oxytetracycline, ampicillin, gentamicin, kanamycin, amikacin, lincomycin and fosfomycin has a significant synergistic effect.
3. The synergistic effect of trimethoprim in combination with polymyxin and kasugamycin can reach 2-32 times.
4. The combined use of trimethoprim, pipemidic acid and norfloxacin has a significant synergistic effect, and the adverse drug reactions are also lower than those of single drug use.
5. The combination of trimethoprim and cefadroxil can enhance the curative effect and delay the generation of bacterial resistance.
6. The concomitant use of dapsone and trimethoprim can increase the blood concentration of both, and the increase of dapsone concentration can increase and aggravate adverse reactions, especially the occurrence of methemoglobinemia.
7. Trimethoprim can interfere with the intrahepatic metabolism of phenytoin, prolong the serum half-life of phenytoin by 50%, and reduce its clearance rate by 30%.
8. The simultaneous use of trimethoprim and procainamide can reduce its renal clearance.
9. Trimethoprim and warfarin can inhibit the metabolism of warfarin and enhance its anticoagulant effect.
Reference
1 Yang Minli, Li Liqing, Lu Jiuru, etc. Potassium permanganate-trimethoprim-sodium thiosulfate chemiluminescence reaction. "Analytical Chemistry", 2001
2 Wang Zhilong, Yan Chengnong, Mei Ping, Fu Li, et al. Synchronized Sensitization Fluorescence Spectroscopic Analysis of Trimethoprim. 2005
You may like
Related articles And Qustion
See also
Lastest Price from Trimethoprim manufacturers

US $2.00-5.00/kg2025-07-24
- CAS:
- 738-70-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100kg

US $0.00-0.00/kg2025-06-03
- CAS:
- 738-70-5
- Min. Order:
- 1kg
- Purity:
- 98.5%-101.0%; BP
- Supply Ability:
- 1000KGS