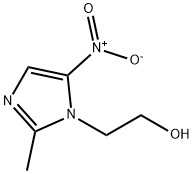
The pharmacopoeia standards of metronidazole
Introduction
Metronidazole, is an antibiotic and antiprotozoal agent. It is mainly used to treat or prevent systemic or local infections caused by anaerobic bacteria, such as anaerobic bacterial infections in the abdominal cavity, digestive tract, female reproductive system, lower respiratory tract, skin and soft tissues, bones and joints, etc. Inflammation, meningeal infections, and colitis caused by antibiotic use are also effective. Tetanus is often treated with tetanus antitoxin (TAT). It can also be used for oral anaerobic infection. On October 27, 2017, the list of carcinogens published by the World Health Organization's International Agency for Research on Cancer was preliminarily sorted for reference, and metronidazole was included in the list of class 2B carcinogens. [1] In January 2020, metronidazole was selected into the second batch of national centralized drug procurement list.
Picture 1 Metronidazole tablets
Resolve resolution
1. It is obtained by the addition of 2-methyl-5-nitroimidazole and ethylene oxide. 2-methyl-5-nitroimidazole was dissolved in formic acid, ethylene oxide was added successively at 30-40°C, and sulfuric acid was added in the middle of the addition[2]. After the addition was completed, the reaction was carried out for 1 h. Formic acid was recovered under reduced pressure, dissolved in water, cooled to 10°C, and filtered. The filtrate was adjusted to pH=10 with sodium hydroxide solution, left to cool, filtered, and washed with water to reconstitute. Recrystallize with water. Activated carbon decolorization, namely metronidazole.
Usage
This product is an anti-amebic drug, an anti-trichomoniasis drug, and an anti-anaerobic bacteria drug. It is a derivative of nitroimidazole. It has the effect of anti-anaerobic protozoa and anaerobic bacteria, and also has radiosensitivity to hypoxic tumor cells.
Pharmacopoeia standards
In the identification of metronidazole, (1) take about 10mg of this product, add 2ml of sodium hydroxide test solution to lukewarm to get a purple-red solution; add dilute hydrochloric acid dropwise to make it acidic and turn yellow, and then add excess sodium hydroxide dropwise The test solution turns orange-red. (2) Take about 0.1g of this product, add 4ml of sulfuric acid solution (3→100), it should be able to dissolve; add 10ml of trinitrophenol test solution, and a yellow precipitate will be formed after standing. (3) Take the solution under the absorption coefficient and measure it by UV-Vis spectrophotometry (Appendix IVA). It has the maximum absorption at the wavelength of 277nm and the minimum absorption at the wavelength of 241nm. (4) The infrared absorption spectrum of this product should be consistent with the control spectrum (spectrum set 112).
In the inspection of metronidazole, the clarity and color of the ethanol solution are taken from this product, dissolved in ethanol and diluted to a solution containing about 5 mg per 1 ml. The solution should be clear; Ⅸ B) comparison, shall not be more concentrated; if the color is developed, it shall not be darker compared with the yellow or yellow-green No. 2 standard colorimetric solution (Appendix IX A). Related substances Protect from light. Take about 100mg of this product, put it in a 100ml measuring bottle, add methanol to dissolve and dilute to the mark, shake well, accurately measure an appropriate amount, dilute with mobile phase to make a solution containing 0.2mg per 1ml, as the test solution; Take about 20mg of 2-methyl-5-nitroimidazole reference substance, put it in a 100ml measuring bottle, add methanol to dissolve and dilute to the mark, shake well, and use it as a reference substance solution. Precisely measure 2ml of the test solution and 1ml of the reference solution respectively, put them in the same 100ml measuring bottle, dilute to the mark with mobile phase, shake well, precisely measure 5ml, put them in a 50ml measuring bottle, and dilute to the mark with mobile phase, Shake well as a control solution. Determined according to high performance liquid chromatography (Appendix V D), with octadecylsilane bonded silica gel as filler, methanol-water (20:80) as mobile phase, detection wavelength at 315nm, and the number of theoretical plates according to metronidazole The calculation of the azole peak should not be less than 2000, and the resolution between the metronidazole peak and the 2-methyl-5-nitroimidazole peak should not be less than 2.0. Take 20 μl of the control solution and inject it into the liquid chromatograph, adjust the detection sensitivity, so that the peak height of the metronidazole chromatographic peak is 10% of the full scale; then precisely measure 20 μl of each of the test solution and the control solution, and inject them into the liquid chromatograph respectively. , record the chromatogram to twice the retention time of the main component peak. If there is a chromatographic peak with the same retention time as 2-methyl-5-nitroimidazole in the chromatogram of the test solution, its peak area shall not be greater than 0.5 times (0.1%) of the peak area of metronidazole in the control solution; The sum of the peak areas shall not be greater than the peak area of metronidazole in the control solution (0.2%). Loss on drying Take this product and dry it at 105 ℃ to constant weight, the weight loss shall not exceed 0.5% (Appendix VIII L). Residue on ignition Take 1.0g of this product and inspect it according to the law (Appendix VIII N), and the residual residue should not exceed 0.1%. Heavy metals Take the residue left under the item of residue on ignition, and inspect it according to the law (Appendix VIII H, the second method). The heavy metal content shall not exceed 10 parts per million.
In the determination of metronidazole content, take about 0.13g of this product, accurately weigh it, add 10ml of glacial acetic acid to dissolve, add 2 drops of naphthol benzyl alcohol indicator solution, and titrate with perchloric acid titration solution (0.1mol/L) to The solution turned green and the titration results were corrected with a blank test. Each 1ml of perchloric acid titration solution (0.1mol/L) is equivalent to 17.12mg of C6H9N3O3. In the category of metronidazole, metronidazole belongs to anti-anaerobic bacteria and anti-trichomoniasis.
Pharmacology
In addition to being used for anti-trichomoniasis and anti-ameba, in recent years, metronidazole has been widely used in anti-anaerobic infection. The nitro group of this product is reduced to amino group in an anaerobic environment and shows the effect of anti-anaerobic bacteria, but it is ineffective against aerobic bacteria or facultative aerobic bacteria. It has good antibacterial effect on the following anaerobic bacteria: ① Bacteroides, including Bacteroides fragilis; ② Clostridium; ③ Clostridium, including Tetanus; ④ Partial Eubacterium; ⑤ Peptococcus and Digestive Streptococcus etc. Oral absorption is good (>80%), oral 250mg or 500mg, 1 to 2 hours serum drug concentration peaks, respectively, 6μg/mL and 12μg/mL. Intravenous infusion of 15 mg/kg of this product, followed by infusion of 7.5 mg/kg every 6 hours, the peak concentration of the drug in plasma reaches a steady state of 25 μg/mL, and the trough concentration can reach 18 μg/mL. This product is widely distributed in the body, and can enter into human saliva, breast milk, pus from liver abscess, and also into cerebrospinal fluid (the concentration in normal human cerebrospinal fluid can reach 50% of blood). In the body, it is metabolized by side chain oxidation or combined with glucuronic acid, and 20% of the drug is not metabolized. Its metabolites also have certain activity. A large amount of metronidazole and its metabolites are excreted in urine (60% to 80% of the total), and a small amount is excreted in feces (6% to 15%). t1/2 is about 8 hours.
Indications
Metronidazole is mainly used to treat or prevent systemic or local infections caused by the above-mentioned anaerobic bacteria, such as anaerobic bacterial infections in the abdominal cavity, digestive tract, female reproductive system, lower respiratory tract, skin and soft tissues, bones and joints, etc. , endocarditis, meningeal infections, and colitis caused by antibiotic use are also effective. Tetanus is often treated with tetanus antitoxin (TAT). It can also be used for oral anaerobic infection.
Dosage
Due to the different dosage forms and specifications of metronidazole, please read the instructions carefully or follow the doctor's advice for usage and dosage.
Adverse reactions
Gastrointestinal reactions are the most common, including nausea, vomiting, loss of appetite, abdominal cramps, and generally do not affect treatment; neurological symptoms include headache, dizziness, occasional paresthesia, limb numbness, ataxia, polyneuritis, etc. Doses can cause convulsions. A few cases of urticaria, flushing, itching, cystitis, dysuria, metallic taste in the mouth and leukopenia, etc., are reversible and recover spontaneously after stopping the drug.
Contraindications
Patients with active central nervous system diseases and blood diseases are disabled. Pregnant women and lactating women are prohibited.
Precautions
(1) Metabolized by the liver, the drug may accumulate in patients with hepatic insufficiency, and the dose should be reduced as appropriate. (2) Sodium intake should be reduced during application, as excessive salt can cause sodium retention. (3) Candida albicans can be induced, and anti-candida drugs can be used together if necessary. (4) It can cause peripheral neuritis and convulsions. In this case, drug withdrawal (or dose reduction) should be considered. (5) It can cause blood changes, leukopenia, etc., attention should be paid. Metabolites of this product can cause dark red urine.
Medicine interactions
(1) This product can slow down the metabolism of oral anticoagulant drugs (such as warfarin, etc.), strengthen its effect, and prolong the prothrombin time. (2) Liver enzyme inhibitors such as cimetidine can decelerate the elimination of this product and increase its efficacy. (3) This product can inhibit acetaldehyde dehydrogenase, so it can strengthen the effect of ethanol, leading to the reaction of disulfiram. Alcohol-containing beverages or medicines should not be used during the medication period and within 1 week after stopping the medication.
Preparation standard
Tablets: 0.2g per tablet. Injection: 50mg (10mL); 100mg (20mL); 500mg (100mL); 1.25g (250mL); 500mg (250mL). Metronidazole Glucose Injection: 250mL, containing 0.5g metronidazole and 12.5g glucose. Suppositories: 0.5g each; 1g. Rectal administration, 0.5g once, 1.5g a day. Maetronidazole vaginal effervescent tablets: 0.2g per tablet. Vaginal administration, 0.2~0.4g once, 7 days as a course of treatment.
Reference
1 Sun Yan. Efficacy analysis of metronidazole suppository combined with estrogen ointment in the treatment of senile vaginitis. 2012
2 Han Yue. Efficacy of estrogen combined with metronidazole in the treatment of atrophic vaginitis. 2013
Related articles And Qustion
Lastest Price from Metronidazole manufacturers

US $0.00-0.00/kg2025-08-15
- CAS:
- 443-48-1
- Min. Order:
- 25kg
- Purity:
- 99%-101%;BP
- Supply Ability:
- 10 tons

US $5.00-0.50/KG2025-05-07
- CAS:
- 443-48-1
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS