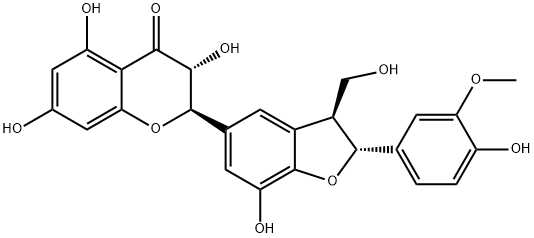
The new research on Silychristin
Introduction
Silymarin is a crude extract of the fruits (cypselae) of milk thistle (Silybum marianum L. Gaertn., Asteraceae), which is used in a plethora of nutraceutical and dietary supplement preparations due to a purported chemo- and hepatoprotective action. Depending on the plant cultivar and extraction method, silymarin contains more than 10 structurally closely related flavonolignans, all biogenetic congeners. The most abundant flavonolignan and the easiest to isolate from silymarin is silybin (silibinin), typically used as a natural mixture of diastereomers silybin A (1a) and silybin B (1b) in a ratio of ca. 1:1 (the numbers denote here natural mixtures of diastereomers of silybin (1) and silychristin (3) and the numbers with letters denote individual diastereomers)[1].

Silychristin is the second most abundant flavonolignan (after silybin) present in the fruits of Silybum marianum. Similar to silybin, natural silychristin from S. marianum consists of two diastereomers: silychristin A and silychristin B. Silychristin A is prevalent in the natural material with a diastereomeric ratio of approximately 95:5 (3a/3b), depending on the source.
The newly research
Topical delivery of silymarin constituents via the skin route.
Silibinin (SB), silydianin (SD), and silychristin (SC) are components of silymarin. These compounds can be used to protect the skin from oxidative stress induced by ultraviolet (UV) irradiation and treat it. To this end, Hung et al. examined the absorption of silymarin constituents via the skin. Transport of SB, SD, and SC under the same thermodynamic activity through and into the skin and the effects of pH were studied in vitro using a Franz diffusion assembly. The lipophilicity increased in the order of SC<SD<SB. Increased lipophilicity of a compound resulted in higher skin deposition but had a minor effect on permeation across the skin in the less-ionized form (pH 8). It is apparent that compounds in the less-ionized form showed higher skin uptake compared to the more-ionized form. Hyperproliferative skin produced by UVB exposure showed increased permeation of silymarin constituents in the less-ionized form, but it did not affect deposition within the skin. The skin disruption and erythema test demonstrated that the topical application of these compounds for up to 24 h caused no apparent skin irritation[2].
Silymarin and silibinin cause G1 and G2–M cell cycle arrest via distinct circuitries in human prostate cancer PC3 cells: a comparison of flavanone silibinin with flavonolignans mixture silymarin.
Here, Deep et al. assessed and compared the anticancer efficacy and associated mechanisms of silymarin and silibinin in human prostate cancer (PCA) PC3 cells; Silymarin and silibinin (50–100 μg/ml) inhibited cell proliferation, induced cell death, and caused G1 and G2–M cell cycle arrest in a dose/time-dependent manner. Molecular studies showed that G1 arrest was associated with a decrease in cyclin D1, cyclin D3, cyclin E, cyclin-dependent kinase (CDK)4, CDK6 and CDK2 protein levels, and CDK2 and CDK4 kinase activity, together with an increase in CDK inhibitors (CDKIs) Kip1/p27 and Cip1/p21. These findings indicate that silymarin and silibinin modulate G1 phase cyclins–CDKs–CDKIs for G1 arrest, and the Chk2–Cdc25C–Cdc2/cyclin B1 pathway for G2–M arrest, together with an altered subcellular localization of critical cell cycle regulators. Overall, comparable effects for both silymarin and silibinin at equal concentrations by weight suggest that silibinin could be a major cell cycle-inhibitory component in silymarin[3].
Flavonolignans reduce the response of blood platelets to collagen.
The primary biological function of platelets is to form hemostatic thrombi that prevent blood loss and maintain vascular integrity. Collagen represents up to 40% of the total protein in the vessel wall, is the primary activator of the platelets' response after tissue injury, and is the only matrix protein that supports platelet adhesion and complete activation[4]. Bijak et al. determined the effects of three significant flavonolignans (silybin, silychristin, and silydianin) on collagen-induced blood platelets' activation, adhesion, aggregation, and secretion of PF-4. They observed that depending on the dose, silychristin and silybin have anti-platelet properties observed as inhibition of collagen-induced activation (formation of blood platelet aggregates and microparticles, as well as decreased expression of P-selectin and activation of integrin αIIbβ3), aggregation, adhesion and secretion of PF-4. These effects highlight the potential of silybin and silychristin as supplementation to prevent primary and secondary thrombotic events wherein excessive blood platelet response to a physiological agonist is observed.
References
[1] David Biedermann*. “Silychristin: Skeletal Alterations and Biological Activities.” Journal of Natural Products 79 12 (2016): 3086–3092.
[2] Chi-feng Hung. “Topical delivery of silymarin constituents via the skin route.” Acta Pharmacologica Sinica 31 1 (2009): 118–126.
[3] G Deep. “Silymarin and silibinin cause G1 and G2-M cell cycle arrest via distinct circuitries in human prostate cancer PC3 cells: a comparison of flavanone silibinin with flavanolignan mixture silymarin.” Oncogene 25 7 (2006): 1053–69.
[4] Michal, et al. "Flavonolignans reduce the response of blood platelet to collagen." International Journal of Biological Macromolecules Structure Function & Interactions (2018).
You may like
See also
Lastest Price from Silychristin manufacturers

US $2.00-5.00/kg2025-07-01
- CAS:
- 33889-69-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100kg
US $0.00/mg2023-02-24
- CAS:
- 33889-69-9
- Min. Order:
- 5mg
- Purity:
- ≥98%(HPLC)
- Supply Ability:
- 10 g