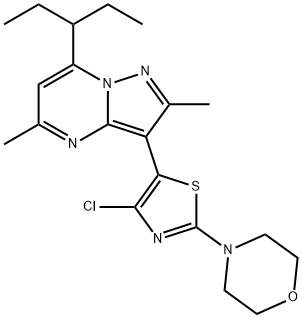
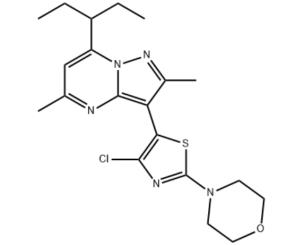
Tildacerfont: A New Frontier in the Treatment of Hypercortisolism
Introduction
In recent years, the pharmaceutical landscape has seen significant advancements in the treatment of endocrine disorders, particularly those involving cortisol dysregulation. One of the most promising developments in this area is Tildacerfont, a novel therapy designed to combat hypercortisolism. This compound has garnered attention due to its unique approach to addressing the root causes of cortisol imbalance through selective receptor antagonism, providing a targeted therapeutic option without the extensive side effects common in broader-spectrum medications. As the incidence of disorders like Cushing's syndrome and congenital adrenal hyperplasia remains high, the introduction of Tildacerfont offers new hope for patients seeking effective management of their condition. This drug not only represents a breakthrough in endocrine therapy but also highlights the strides being made in drug development and personalized medicine[1].
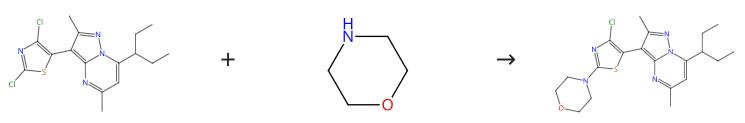
Figure 1: Synthesis path of Tildacerfont
Mechanism of Action
Tildacerfont operates primarily as a glucocorticoid receptor antagonist. Its mechanism is distinctively designed to target and modulate the receptor interactions that are crucial in the pathophysiology of cortisol excess. By binding competitively to glucocorticoid receptors, Tildacerfont prevents cortisol from exerting its effects on the target tissues. This mode of action is particularly beneficial in diseases where cortisol is pathologically elevated, thus providing a targeted approach that minimizes the systemic side effects typically associated with other treatment modalities.
The pharmacodynamics of Tildacerfont are characterized by a high affinity for the glucocorticoid receptor, coupled with a slow dissociation rate, which enhances its efficacy in sustained cortisol suppression. Additionally, its selectivity ensures that other steroid receptors, such as mineralocorticoid receptors, remain largely unaffected, thereby reducing the risk of off-target effects.
Applications
The primary application of Tildacerfont is in the treatment of conditions characterized by cortisol excess, such as Cushing's syndrome and congenital adrenal hyperplasia (CAH). These conditions, which can be debilitating and life-threatening, have historically been managed with treatments that range from surgery to systemic steroid use, each with its own set of limitations and side effects.
Tildacerfont represents a significant step forward, offering a non-invasive alternative that can be used either as a monotherapy or in conjunction with other treatments. Clinical trials have demonstrated its potential to significantly reduce the clinical signs of hypercortisolism, improve patient quality of life, and decrease the need for additional pharmacological intervention.
Furthermore, ongoing research suggests potential applications beyond traditional hypercortisolism. Studies are exploring its use in other disorders where cortisol plays a role, such as certain psychiatric and inflammatory diseases, indicating a broad potential impact of this therapeutic agent.
Storage
Proper storage of Tildacerfont is crucial to maintaining its chemical integrity and therapeutic efficacy. The compound should be stored at controlled room temperatures, typically between 20°C to 25°C (68°F to 77°F), with allowances for brief excursions that are customary in pharmacies and hospitals. The product must be protected from light and moisture, and stored in its original packaging to prevent contamination. As with all pharmaceuticals, it should be kept out of reach of children and managed according to local regulatory guidelines regarding controlled substances. Additionally, it is imperative to monitor the expiration dates and handle the drug with care during transport to ensure stability. Specific storage requirements may also include avoiding certain chemicals or environments that could degrade the compound, further ensuring that its therapeutic properties are preserved until administration[2].
Conclusion
Tildacerfont marks a significant advancement in the pharmacological management of diseases associated with cortisol excess. Its development reflects both an evolution in the understanding of the underlying biochemistry of cortisol-related disorders and a stride forward in patient care. As further research elucidates additional applications and long-term outcomes, Tildacerfont is poised to become a cornerstone in the treatment of hypercortisolism, offering hope to those afflicted by these challenging conditions.
References
[1]Sarafoglou K, Barnes C N, Huang M, et al. Tildacerfont in adults with classic congenital adrenal hyperplasia: results from two phase 2 studies[J]. The Journal of Clinical Endocrinology & Metabolism, 2021, 106(11): e4666-e4679.
[2]Barnes C, Offman E, Darago N, et al. Dose escalating and bioavailability Phase 1 studies assessing safety and tolerability and pharmacokinetics of tildacerfont, a small-molecule CRF1 receptor antagonist[J]. Journal of the Endocrine Society, 2021, 5(Suppl 1): A67.