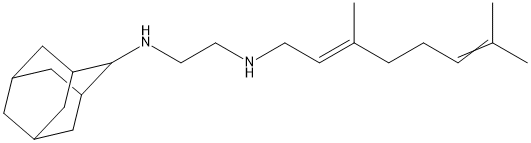
The introduction of Cenobamate (SQ 109)
Introduction
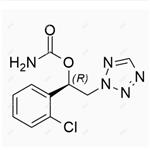
Cenobamate ((R)-1-(2-chlorophenyl)-2-(2H-tetrazol-2-yl)ethyl), also known as YKP3089 or SQ 109, was developed by SK Life Sciences. Cenobamate is the latest approved antiepileptic drug in focal epilepsy, and its mode of action is thought to be mediated by blocking voltage-gated sodium channels and interacting with the GABAergic system[1].
Biological action
Regardless of its primary purpose, cenobamate is a promising neuroprotective agent as a blocker of voltage-gated sodium channels and positive modulator of GABAa receptors. Moreover, the activation of the PI3K/Akt-CREB-BDNF pathway leads to the increase of anti-apoptotic factor levels and the decrease of pro-apoptotic factor levels, which induce inhibition of apoptosis and increase neuron survival[2]. Similarly to riluzole, cenobamate could be an essential part of a perioperative procedure in neurosurgery, decreasing the occurrence of neurological deficits.
Synthesis method
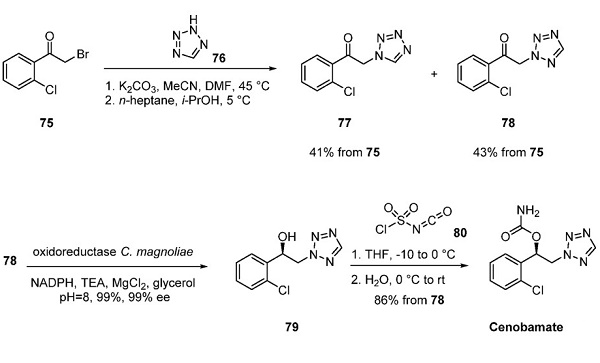
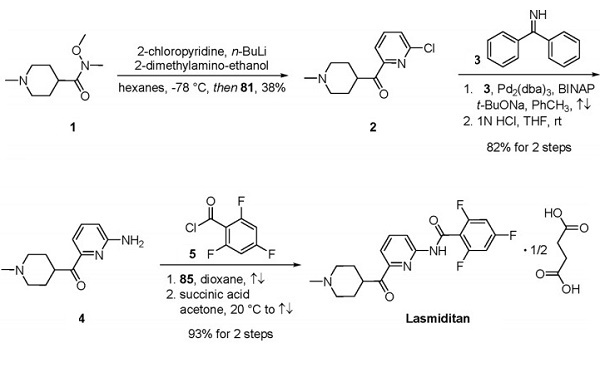
To date, a large-scale synthesis of cenobamate has not been explicitly reported. Patent literature from SK Life Sciences indicated that the drug was accessible three steps from α- bromo ketone 75[3]. Treatment of 75 with 1Htetrazole (76) in DMF in the presence of K2CO3 at room temperature resulted in a 1:1 mixture of tetrazole regioisomers 77 and 78. Subsequent crystallization of the mixture from chilled heptane gave a 43% yield of the desired tetrazole regioisomer 78, while a 41% yield of the undesired tetrazole regioisomer 77 was recovered. Although asymmetric catalytic hydrogenation to access chiral alcohol 79 could be envisaged, SK Life Sciences described an elegant enantioselective enzymatic reduction. Tetrazole 78 was treated with RB791 cells transfected with the expression constructs pET21-MIX coding for the oxidoreductase from Candida magnoliae for 48 h at room temperature. Exposure to the oxidoreductase resulted in a 99% conversion of ketone 78 to chiral alcohol 79 with >99% ee on a 50 g scale. After aqueous workup, crude chiral alcohol 79 was treated with chlorosulfonyl isocyanate (80) in THF. Cenobamate was obtained in 86% yield from tetrazole 78 after water quench and crystallization.
References
[1] Adam Strzelczyk. “Cenobamate for the treatment of focal epilepsies.” Expert Opinion on Pharmacotherapy 21 18 (2020): 2215–2223.
[2] Michał Wiciński, Bartosz Malinowski, Oskar Puk. “Cenobamate: Neuroprotective Potential of a New Antiepileptic Drug.” Neurochemical Research 46 3 (2020): 439–446.
[3] Andrew C. Flick. “Synthetic Approaches to the New Drugs Approved during 2019.” Journal of Medicinal Chemistry 64 7 (2021): 3604–3657.
You may like
See also
US $0.00-0.00/kg2025-04-16
- CAS:
- 502487-67-4
- Min. Order:
- 5kg
- Purity:
- 98%-102%
- Supply Ability:
- 200kg
US $0.00/mg2025-03-22
- CAS:
- Min. Order:
- 10mg
- Purity:
- 0.98
- Supply Ability:
- 5g