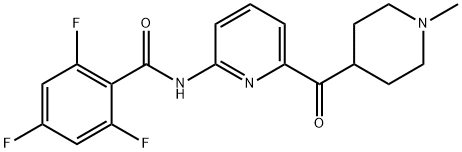
How Lasmiditan works in treating migraine
Description
Lasmiditan, or COL-144, is an orally bioavailable selective serotonin receptor agonist (SSRA) discovered by Eli Lilly and Company and licensed to CoLucid Pharmaceuticals before Eli Lilly acquired CoLucid. Approved by the USFDA for the acute treatment of migraine with or without aura in adults, the once-daily-dosed drug is the first in a novel class of drugs called “titans.”
Biological function
Lasmiditan (2,4,6-trifluor-N-6-[(1-methyl-piperidin-4-yl)carbonyl]pyridin-2-yl-benzamid) is a highly selective 5-HT1F receptor agonist. The 5-HT1F receptor is expressed on the presynaptic surface of central and peripheral trigeminal sensory neurons, and its activation does not lead to vasoconstriction. These properties make the receptor an ideal target for new acute migraine treatments[1]. The efficacy of lasmiditan to block CGRP release via the 5-HT1F receptor has been studied in vitro and animal models in samples of dura mater, trigeminal ganglion, and trigeminal nucleus caudalis of rodents. Lasmiditan blocked the release of CGRP in all tissues in a magnitude comparable to the blocking activity of sumatriptan in the identical setting. In vivo, lasmiditan infusion inhibits neurogenic dural vasodilation induced through i.v. capsaicin and electrical trigeminal ganglion stimulation at lower doses compared to sumatriptan. However, lasmiditan could not attenuate non-neurogenic vasodilation in dura mater in response to exogenous CGRP infusion, which implies a presynaptic mechanism of action; that is, inhibition of endogenous CGRP release.
Synthesis method
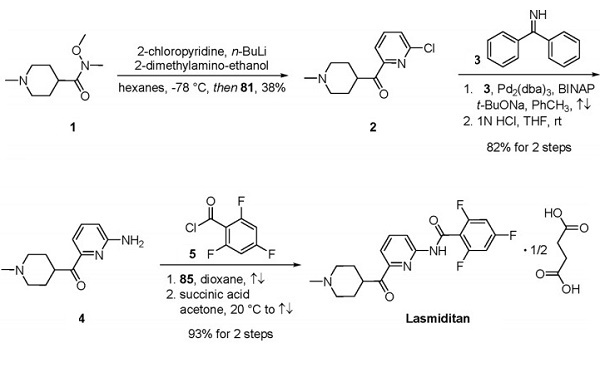
A robust synthesis of the drug was first described by researchers at Eli Lilly and is depicted above [2]. Lithiation of 2-chloropyridine and subjection to Weinreb amide 1 delivered methylpiperidine 2 in 38% yield. A Buchwald−Hartwig reaction employing benzophenone imine (3) followed by acidic deprotection provided primary aryl amine 4 in 82% yield over two steps. Amidation of 4 with benzoyl chloride 5 followed by subjection to succinic acid furnished lasmiditan as the hemisuccinate salt in excellent yield over two steps.
References
[1] Jasper Mecklenburg. “The potential of lasmiditan in migraine.” Therapeutic Advances in Neurological Disorders 13 (2020): 1756286420967847.
[2] Andrew C. Flick. “Synthetic Approaches to the New Drugs Approved during 2019.” Journal of Medicinal Chemistry 64 7 (2021): 3604–3657.
See also
Lastest Price from LasMiditan manufacturers

US $0.00-0.00/g2024-11-01
- CAS:
- 439239-90-4
- Min. Order:
- 1g
- Purity:
- 98%
- Supply Ability:
- 1000kgs

US $15.00-10.00/KG2021-07-13
- CAS:
- 439239-90-4
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton