The synthesis method of Lemborexant
Introduction
Lemborexant is an orally administered dual orexin receptor (OXR) antagonist developed by Eisai for the treatment of adults with insomnia. Lemborexant received its first approval from the USFDA, with subsequent approval in Japan in January 2020. Unlike many other pharmaceutical treatments for insomnia involving γ-aminobutyric acid or monoamine agonists, lemborexant exhibits reversible competitive antagonism of OXR1 and OXR2. Orexin peptides help regulate the sleep-wake cycle such that the orexin neurons are active during waking hours and inactive during sleep.
Synthesis method
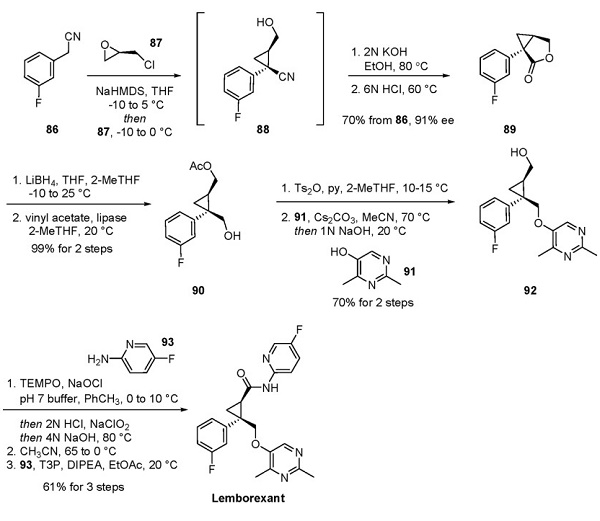
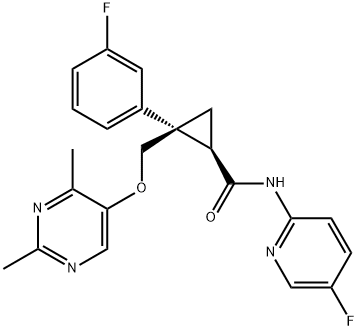
Lemborexant analogs and their synthetic approaches were first reported in 2015. However, a synthesis of lemborexant on a 200 g scale was disclosed in a 2013 patent, and this approach is depicted below[1].
Deprotonation of aryl acetonitrile 86 with NaHMDS and subsequent exposure to (R)-epichlorohydrin (87) afforded cyclopropane 88, which was immediately converted in situ to the carboxylic acid in the presence of base. Upon acidification, the acid intermediate underwent lactonization to produce 89 in 70% yield with 91% ee. Reduction of lactone 89 to the corresponding diol with LiBH4 followed by selective enzymatic acetylation of the less hindered alcohol furnished alcohol 90 in essentially quantitative yield. Tosylation followed by SN2 displacement with pyrimidinol 91 was accompanied by in situ cleavage of the acetate group during workup, providing alcohol 92 in 70% yield over two steps. Next, a one-pot, two-step oxidation of the primary alcohol within 92 to the carboxylic acid was facilitated by TEMPO and NaOCl, followed by treatment with NaClO2 and, finally, a pH adjustment. Recrystallization from acetonitrile occurred before T3P-mediated amidation with aminopyridine 93 to provide lemborexant in 61% yield over three steps
References
[1] Andrew C. Flick. “Synthetic Approaches to the New Drugs Approved during 2019.” Journal of Medicinal Chemistry 64 7 (2021): 3604–3657.
Related articles And Qustion
See also

US $0.00-0.00/g2025-09-23
- CAS:
- 1369764-02-2
- Min. Order:
- 10g
- Purity:
- 99%
- Supply Ability:
- 50kg

US $0.00-0.00/G2025-09-11
- CAS:
- 1369764-02-2
- Min. Order:
- 1G
- Purity:
- 99%
- Supply Ability:
- KG