Lemborexant: A Dual Orexin Receptor Antagonist with Favorable Safety and Pharmacokinetic Profiles
General Description
Lemborexant, a dual orexin receptor antagonist, facilitates sleep onset and maintenance while allowing normal awakening to external stimuli. It exhibits high selectivity for OXR1 and OXR2 and has shown positive effects on promoting sleep in animal studies. In terms of safety, clinical data indicate good tolerability in adults, with adverse events generally mild and similar to placebo. The most common reasons for discontinuation were somnolence and nightmares, with some patients reporting sleep-related behaviors. Lemborexant did not increase apneic events or oxygen desaturation in mild obstructive sleep apnea patients, but caution is advised for those with compromised respiratory function. Additionally, prescribers should educate patients about potential side effects, particularly in older individuals. Pharmacokinetic data of Lemborexan reveal rapid absorption, metabolism by CYP3A4, and potential interactions with other drugs, necessitating careful consideration during co-administration.

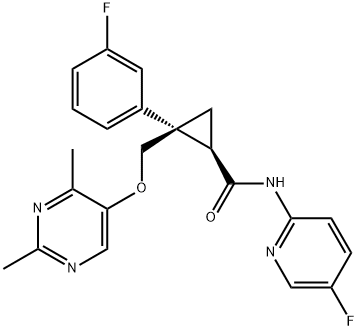
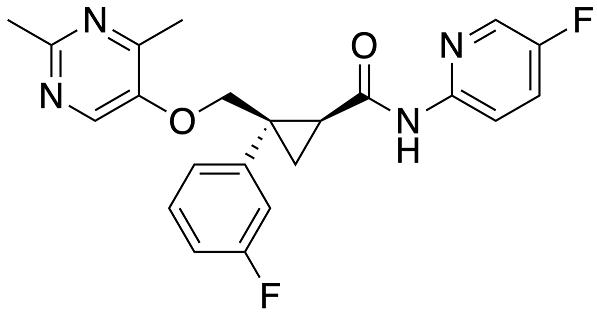
Figure 1. Lemborexant
Dual Orexin Receptor Antagonism
Lemborexant exerts its pharmacodynamic effects through dual orexin receptor (OXR) antagonism, acting as a competitive antagonist at both OXR1 and OXR2. In vitro studies have shown that lemborexant and its major metabolite M10 bind to OXR1 and OXR2 with high affinity, interfering with orexin neurotransmission to facilitate sleep onset and maintenance, while allowing for normal awakening to external stimuli. The drug displays rapid binding and dissociation kinetics from OXRs and exhibits high selectivity for OXR1 and OXR2 compared to other physiologically important receptors, transporters, and ion channels. In animal studies, lemborexant significantly reduces wakefulness time and increases both REM and non-REM sleep without altering the REM sleep ratio. Moreover, it has been demonstrated that the drug's effect on sleep promotion is absent in an orexin neuron-deficient mouse model, supporting its dual mechanism of action on OXRs. In terms of safety, a supratherapeutic dose of lemborexant in healthy volunteers did not lead to clinically relevant prolongation of the Fridericia’s corrected QT interval, based on concentration-QTcF analysis of clinical studies. Additionally, in animal models, lemborexant did not potentiate the sedative effects of ethanol or impair motor coordination. These findings suggest that lemborexant's pharmacodynamic profile supports its potential as a safe and effective treatment for sleep disorders. 1
Pharmacokinetics
Lemborexant's pharmacokinetics involve rapid absorption after oral administration, with a median time to peak plasma concentration (tmax) of approximately 1-3 hours. The absorption rate and extent can be affected by food intake, particularly high-fat, high-calorie meals, which delay the tmax by 2 hours and alter absorption parameters. The drug is highly bound to plasma proteins (around 94%) and has a large volume of distribution of 1970 L. Lemborexant is primarily metabolized by CYP3A4 and to a lesser extent by CYP3A5, with the major circulating metabolite being M10. Elimination occurs mainly through feces (57.4%) and urine (29.1%), with a small percentage excreted unchanged. The effective half-life of lemborexant varies with dosage, being approximately 17 hours for a 5 mg dose and 19 hours for a 10 mg dose. Factors such as age, sex, race/ethnicity, and body mass index do not significantly impact lemborexant's pharmacokinetics. Dosage adjustments are necessary for patients with hepatic impairment, while renal impairment may increase the risk of somnolence. Regarding drug interactions, lemborexant has the potential to interact with CYP3A4 inducers and inhibitors, necessitating caution when co-administered with certain medications. Weak CYP3A4 inhibitors have a modest effect on lemborexant exposure, whereas strong inducers or inhibitors can significantly alter its levels. Clinically relevant interactions have been observed with specific drugs, highlighting the importance of careful consideration when prescribing lemborexant in conjunction with other medications. 2
Safety Profile and Tolerability
Lemborexant, an oral medication prescribed for insomnia disorder, has shown good tolerability in adults during up to 12 months of treatment. Adverse events were generally mild and similar to those experienced with a placebo. A pooled safety analysis of studies SUNRISE 1 and SUNRISE 2 revealed that only a small percentage of patients discontinued treatment due to adverse reactions, with somnolence and nightmares being the most common reasons. During the initial 30 days of treatment, the most frequent adverse reactions leading to discontinuation were somnolence and nightmares. Over a 6-month period, somnolence, nightmares, and palpitations were the primary reasons for discontinuation. Some patients reported experiencing sleep paralysis, hypnagogic hallucinations, and even complex sleep behavior, such as sleep-walking or sleep-driving, particularly with the 10 mg dose. Notably, Lemborexant did not increase apneic events or oxygen desaturation in patients with mild obstructive sleep apnea. However, caution is advised for individuals with compromised respiratory function. In older individuals, Lemborexant may impair balance, attention, and memory, highlighting the importance of understanding potential side effects before use. Additionally, prescribers should educate patients about the risks of sleep paralysis, hallucinations, and other potential symptoms associated with Lemborexant treatment. 3
Reference
1. Eisai Inc. DAYVIGOTM (lemborexant): US prescibing information. 2019.
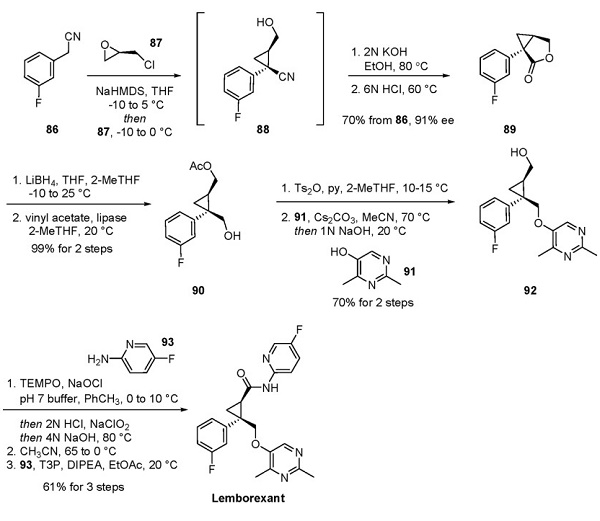
2. Yoshida Y, Naoe Y, Terauchi T, et al. Discovery of (1R,2S)2-{[(2,4-Dimethylpyr imidin-5-yl)oxy]methyl}-2-(3fluorophenyl)-N-(5-fluoropyridin-2-yl)cyclopropanecarboxamide (E2006): a potent and efficacious oral orexin receptor antagonist. J Med Chem. 2015; 58(11): 4648–4664.
3. Scott LJ. Lemborexant: First Approval. Drugs. 2020; 80(4): 425-432.
Related articles And Qustion

US $0.00-0.00/g2025-09-23
- CAS:
- 1369764-02-2
- Min. Order:
- 10g
- Purity:
- 99%
- Supply Ability:
- 50kg

US $0.00-0.00/G2025-09-11
- CAS:
- 1369764-02-2
- Min. Order:
- 1G
- Purity:
- 99%
- Supply Ability:
- KG