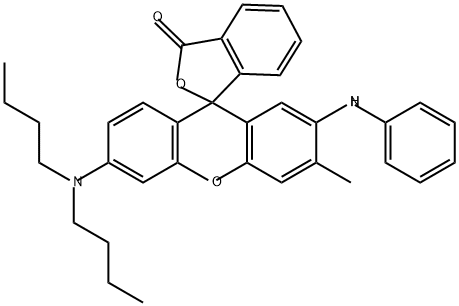
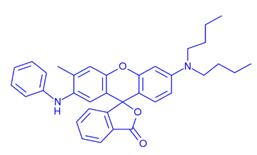
2-Anilino-6-dibutylamino-3-methylfluoran: Overview, Applications in Light-Driven Phase Change Materials System and Preparation Method
Overview
2-Anilino-6-dibutylamino-3-methylfluoran is almost colorless in itself, is extremely stable in the air, has no sublimation property and has no spontaneous color development (background fogging), and is extremely well soluble in an organic solvent. The color developer rapidly develops a black color, and the color image has excellent light resistance and moisture resistance.
Applications in Light-Driven Phase Change Materials System
2-Anilino-6-dibutylamino-3-methylfluoran plays a crucial role in the development of temperature-controlled light-driven phase change materials (PCMs) systems. A novel PCM system was devised by incorporating a thermochromic compound like 2-Anilino-6-dibutylamino-3-methylfluoran. In this study, thermochromic phase change materials (TC-PCMs) were formulated by blending 2-Anilino-6-dibutylamino-3-methylfluoran and bisphenol A with 1-hexadecanol (1-HD) in different ratios.
Preparation Method
To obtain the objective high-quality high-melting compound in a high yield. CONSTITUTION: The production process is one for producing 2-anilino-3-methyl- -6-dibutylaminofluoran, having a melting point in the range of 179-186 deg.C comprising the steps of: reacting 2-(4'-dibutylamino-2'-hydroxybenzoyl)benzoic acid with 1-1.2mol, per mol of the former compound, of 4-methoxy-2-methyl- diphenylamine at 0-30 deg.C in concentrated sulfuric acid of 96% or above and filtering off the phthalide-containing precipitates formed by introducing the reaction solution into ice water, and treating the precipitates in a mixture of an aqueous sodium hydroxide solution and an organic solvent which are separable from each other at 8-100 ℃ for 1-3hr to cyclize the phthalide in the precipitates, wherein the amount of the sodium hydroxide excluding that used to neutralize the precipitates is 0.2-0.4mol per mol of the 2-(4-'-dibutylamino-2'- hydroxybenzoyl)benzoic acid.1
Reference
1. JPH0860002A
You may like
Related articles And Qustion
See also
Lastest Price from 2-Anilino-6-dibutylamino-3-methylfluoran manufacturers

US $0.00/Kg/Drum2025-04-21
- CAS:
- 89331-94-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500mt/year

US $0.00-0.00/KG2025-04-15
- CAS:
- 89331-94-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg