Dasatinib: Mechanism of action and Safety
Introduction
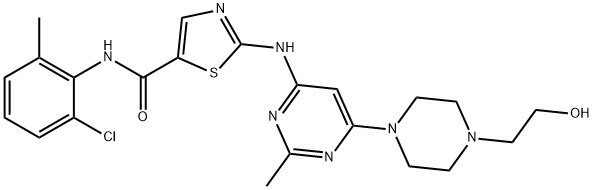
Dasatinib, a tyrosine kinase inhibitor, primarily treats chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia. Oral administration of dasatinib is convenient and promotes improved quality of life for patients. However, compared to conventional intravenous treatment, oral use introduces the risk of variable drug exposure, which may translate into decreased drug efficacy or variable safety[1–2].

Dasatinib is a potent second-generation TKI, 325 times more potent than imatinib in inhibiting the BCR::ABL1 tyrosine kinase in vitro and inhibiting the Src family kinases. In the5-year follow-up of the Dasatinib versus Imatinib study in treatment-naïve chronic myeloid leukemia patients. Trial dasatinib was established as a standard of care, producing faster and deeper molecular responses compared with imatinib, but it did not improve survival. Dasatinib 100 mg once daily was associated with high optimal response rates defined byBCR::ABL1transcript levels≤10% at 3 months compared with imatinib (84% vs. 64%;p< .0001). Early clinical trials demonstrated the activity of dasatinib at lower doses with a favorable safety profile. These findings were confirmed in a randomized trial of different dosing schedules of dasatinib, showing comparable efficacy and less toxicity with the lower doses. Dose adjustments of dasatinib for adverse events were not associated with reduced efficacy or outcome compromise with long-term follow-up[3].
Mechanism of action
Dasatinib is a tyrosine kinase inhibitor with several targets. At nanomolar concentrations, it inhibits BCR-ABL, SRC family (SRC, LCK, YES, FYN), c-KIT, EPHA2, and PDGFRβ. In patients with chronic myeloid leukemia (CML), the tyrosine kinase activity of BCR-ABL is deregulated, leading to the growth, proliferation, and survival of cancerous hematopoietic cells. Dasatinib binds to the active and inactive conformation of the ABL kinase domain with a higher affinity than imatinib. Dasatinib inhibits cell growth in chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL) cell lines overexpressing BCR-ABL. Also, dasatinib has in vitro activity against leukemic cell lines that are either sensitive or resistant to imatinib. It has been suggested that dasatinib can overcome imatinib resistance caused by BCR-ABL kinase domain mutations because it does not require interaction with some of the residues involved in those mutations.
Safety
FDA label warnings for the clinical use of dasatinib include cardiac dysfunction, pulmonary arterial hypertension, and QT prolongation. Cardiovascular and other toxicities associated with dasatinib and the other BCR-ABL1-targeted tyrosine kinase inhibitors have been reviewed. A side effect of dasatinib is proteinuria and, rarely, nephrotic syndrome. Dasatinib is a second-generation TKI used in CML that targets both BCR-ABL and the Src family of kinases involved in VEGF signaling. Podocytes produce VEGF and help maintain basement membrane function. This disruption of VEGF is thought to lead to proteinuria, and the severity is dose-dependent [4]. While nephrotic syndrome is uncommon, minimal change disease and focal segmental glomerulosclerosis have been reported. Patients will typically present with edema and hyperlipidemia. Most patients will recover with the removal of the medication. Patients who develop proteinuria can often be switched to first-generation TKIs, which do not affect VEGF.
References
[1] “63 Dasatinib-Induced Minimal Change Disease.” American Journal of Kidney Diseases 75 4 (2020): Page 554.
[2] Jiří Hofmann. “Dasatinib anhydrate containing oral formulation improves variability and bioavailability in humans.” Leukemia 37 12 (2023): 2486–2492.
[3] Elias Jabbour. “Low-dose dasatinib 50 mg/day versus standard-dose dasatinib 100 mg/day as frontline therapy in chronic myeloid leukemia in chronic phase: A propensity score analysis.” American Journal of Hematology 97 11 (2022): 1413–1418.
[4] Brian B Hasinoff, Daywin Patel. “Mechanisms of the Cardiac Myocyte-Damaging Effects of Dasatinib.” Cardiovascular Toxicology 20 4 (2020): 380–389.
Related articles And Qustion
See also
Lastest Price from Dasatinib manufacturers

US $0.00/g2025-04-21
- CAS:
- 302962-49-8
- Min. Order:
- 1g
- Purity:
- 98%min
- Supply Ability:
- 1000g

US $0.00/KG2025-04-21
- CAS:
- 302962-49-8
- Min. Order:
- 2KG
- Purity:
- USP26, EP VI / GMP DMF
- Supply Ability:
- 20tons