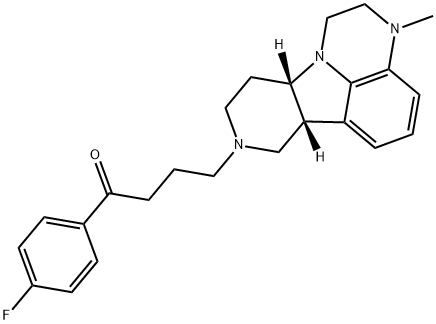
A first-in-class potent 5-HT2A antagonist: Lumateperone
Introduction
Lumateperone, also known as ITI-007, is a first-in-class potent 5-HT2A antagonist, postsynaptic D2 antagonist, and inhibitor of serotonin transport developed by Intra-Cellular Therapies under a license from Bristol-Myers Squibb. Lumateperone is a novel, orally available agent for the treatment of schizophrenia and other neuropsychiatric and neurological disorders. The drug is also under clinical development for bipolar depression, behavioral disorders associated with dementia and Alzheimer's disease, sleep maintenance insomnia, and major depressive disorders[1].
Mechanism of action
Like other SGAs, lumateperone is an antagonist of 5-HT2A and D2 receptors. Lumateperone has a high affinity for 5-HT2A, moderate affinity for D2, and low affinity for α-1 and histamine-1 receptors. Because of lumateperone's relatively low binding affinity for α-1 and histamine-1 receptors, it is associated with fewer adverse effects related to antagonism of these receptors compared with some other SGAs such as aripiprazole, quetiapine, clozapine, and olanzapine. At D2 receptors, lumateperone is a presynaptic partial agonist and postsynaptic antagonist[2]. The receptor binding of this agent is many times (~60×) more potent for the 5-HT2A receptor than the D2 receptor, which may contribute to the relatively low risk for EPS observed in the clinical trials.
Synthesis method
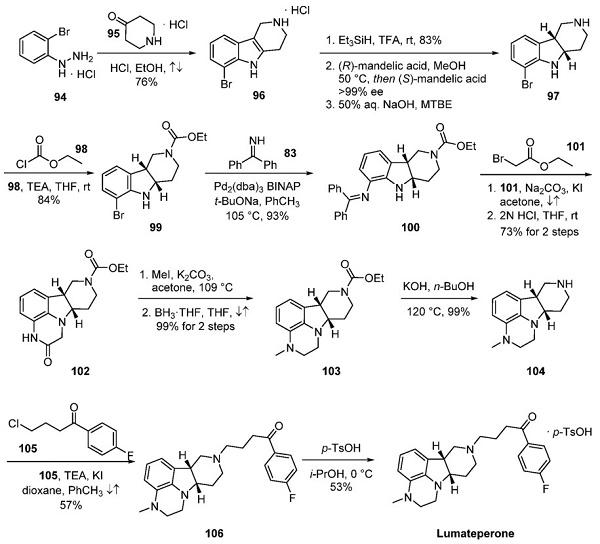
A concise, scalable route to lumateperone and structurally related analogs has been described. Starting with hydrazine 94, a Fischer indole synthesis with ketone 95 gave tricyclic indole 96[3]. Reduction with triethylsilyl hydride, treatment of the resulting product with (R)-mandelic acid in MeOH, subsequent formation of the (S)- mandelic acid diastereomeric salt, and free-basing with aqueous NaOH afforded chirally pure cis-indoline 97. Protection of the amine and subsequent Buchwald−Hartwig reaction with 83 furnished cyclization precursor 100. NAlkylation with ethyl bromoacetate (101) followed by hydrolysis of the diphenylamine gave tetracycle 102 via spontaneous ring closure. A second N-alkylation followed by reduction of the carbonyl with borane in THF gave piperazine 103. Carbamate hydrolysis and a final N-alkylation with 4- chloro-1-(4-fluorophenyl)butan-1-one (105) provided the fully elaborated cis tetracycle 106. The free base of 106 was dissolved in isopropanol and treated with a solution of ptoluenesulfonic acid to provide lumateperone tosylate.
References
[1] Jessica Greenwood. “Lumateperone: A Novel Antipsychotic for Schizophrenia.” Annals of Pharmacotherapy 55 1 (2021): 98–104.
[2] Blair, Hannah A. “Lumateperone: First Approval.” Drugs 80 4 (2020): 417–423.
[3] Andrew C. Flick. “Synthetic Approaches to the New Drugs Approved during 2019.” Journal of Medicinal Chemistry 64 7 (2021): 3604–3657.
See also
US $0.00/mg2025-03-27
- CAS:
- 313368-91-1
- Min. Order:
- 10mg
- Purity:
- 0.98
- Supply Ability:
- 5g

US $0.00/g2024-11-19
- CAS:
- 313368-91-1
- Min. Order:
- 1g
- Purity:
- 98% HPLC
- Supply Ability:
- 100g