The introduction of 4-bromoisoindolin-1-one
General description
The 4-bromoisoindolin-1-one, with the CAS No: 337536-15-9, is also known as 4-bromo-2,3-dihydroisoindol-1-one. This chemical’s molecular formula is C8H6BrNO and molecular weight is 212.043. Its boiling point is 448.9 ± 45.0 ° C at 760 mm Hg,and its flash point is 225.3 ± 28.7 °C. The 4-bromoisoindolin-1-one is a kind of synthetic intermediate. Its structure is as follows:
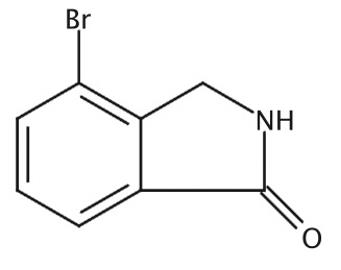
Figure 1 Structure of 4-bromoisoindolin-1-one.
Application of 4-bromoisoindolin-1-one
4-bromoisoindolin-1-one is used to synthesize Vaniprevir [1]. It is an inhibitor of HCV NS3/4A. It reversibly binds to the large peptide ring of NS3/4A and inhibits the decomposition of HCV precursor protein by NS3/4A, thus inhibiting the virus replication cycle. This drug is an antihepatitis C drug approved for marketing by the Ministry of Health, Welfare and Labor (MHLff) of Japan on September 26, 2014. The drug is developed, produced and sold by MSD. The drug is approved forTreatment of genotype 1 chronic hepatitis C infection and viremia. Furthermore, 4-bromoisoindolin-1-one, as a kind of synthetic intermediate, is used to synthesize isoindolinones, widely existed in pharmaceuticals and complex natural products [2]. For example, indoprofen (an anti-inflammatiory), lennoxamine (an anticancer), 9b-phenyl-2,3-dihydrothianolo[2,3,a]-isoindol-5(9bH)-one (a HIV-1 reverse transcriptase inhibitor), pestalacholoride A (an antifungal metabolite), chlortalidoneis (a diuretic drug), garenoxacin (an antibiotic). In this regard, a plenty of approaches have been developed for the synthesis of 4-bromoisoindolin-1-one, both non-asymmetric and asymmetric. 4-bromoisoindolin-1-one can also be synthesize N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), a prototypical and intensely-studied kinase inhibitor [3]. It was one of the first non-natural, synthetic inhibitors that competitively inhibited the binding of ATP to the structurally conserved binding domain of cAMP-dependent protein kinase (PKA). The binding mode of H-89 to PKA has been studied in great detail at the atomic level using crystallization studies. This contributed to the understanding of kinase function and provided general principles to develop drug-like kinase inhibitors. The isoquinoline sulfonamide mi- mics the binding mode of adenosine. The nitrogen of the isoquinoline ring forms a crucial H-bond bridge to the backbone of Val-123, located in the hinge region of PKA.
Chemical synthesis of 4-bromoisoindolin-1-one
The 4-bromoisoindolin-1-one can be synthesized by 3 steps according to the previous work [4]. Briefly, suspension of 3-bromo-2-methylbenzoic acid (9.9 g, 46 mmol) in thionyl chloride (20 mL) was heated to 60 °C for 1 hour, cooled to room temperature, and concentrated. The residue was suspended in 50 mL of methanol, cooled to 0 °C, treated slowly with triethylamine (12.7 mL, 92 mmol), warmed to room temperature, and concentrated. The residue was partitioned between ethyl acetate and water and the organic phase was washed with saturated NaHCO3 and brine, dried (MgSO4), filtered, and concentrated to give of the desired product. Yield 7.39g. Rf= 0.5 (10% ethyl acetate/hexanes). Suspension of Example 1A (7.4 g, 32.3 mmol), NBS (6.9 g, 38.8 mmol), and benzoyl peroxide (0.782 g, 3.2 mmol) in benzene (100 mL) was stirred at reflux for 5 hours, cooled to 0 °C, and filtered. The solid was washed with diethyl ether and the filtrate was washed sequentially with 10% Na2S2O3 (2 x 20 mL), and brine, dried (MgSO4), filtered, and concentrated. The residue was purified by silica gel chromatography with 5 to 10% ethyl acetate/hexanes to give of the desired product. Yield 9.32g. Rf = 0.2 (5% ethyl acetate/hexanes). A solution of Example 1B (8.3 g, 26.9mmol) in THF (100 mL) was treated dropwise with concentrated NH4OH (9 mL, 135 mmol) stirred at room temperature for 2 days, diluted with 30 mL water, cooled to 0 °C, and filtered. The filter cake was washed with water and 10 ethyl acetate and dried to give of the desired product. Yield 3.34 g. MS (ESI(+)) m/e 212 (M+H)+.
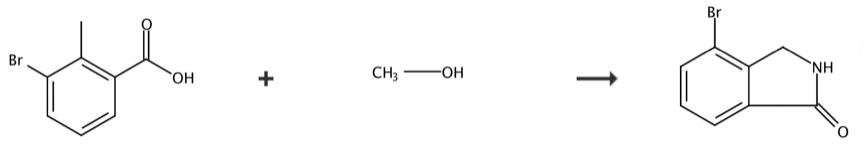
Figure 2 Chemical synthesis of 4-bromoisoindolin-1-one.
References
[1]Zhu, Y et al. Process for synthesis of HCV virus NS3/4A inhibitor intermediate. CN 109678787, 26 Apr 2019.
[2]Ling, F et al. Traceless Directing Group Assisted Cobalt-Catalyzed C-H Carbonylation of Benzylamines. Advanced Synthesis Catalysis, 2017, 359 (21): 3707-3712.
[3]Grimm, SH et al. Comprehensive structure-activity-relationship of azaindoles as highly potent FLT3 inhibitors. Bioorganic Medicinal Chemistry, 2019,27: 692–699.
[4]Matulenko et al. Isoindolinone derivatives as protein kinase inhibitors and their preparation and use for the treatment of pain. PCT Int. Appl., 2012061602, 10 May 2012.


![189028-95-3 (4S)- 3-[(5R)-5-(4-fluorophenyl)-5-hydroxypentanoyl]-4-pheny-1,3-oxazolidin-2-onebiosynthesisintermediate](/NewsImg/2022-12-22/6380731221119374044108082.jpg)