The Extraction method of Hesperidin
Introduction
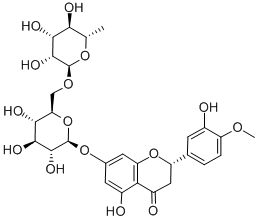
Hesperidin (3,5,7-trihydroxyflavanone 7-rhamnoglucoside, hesperetin-7-O-rutino- side) belongs to flavanone compounds, one of the flavonoids subclasses. It is a disaccharide derivative that consists of hesperetin substituted by a 6-O-(alpha-L-rhamnopyranosyl)-beta-D-glucopyranosyl moiety at position 7 via a glycosidic linkage. It has been recently extensively evaluated for its health-promoting and pharmacological effects. It is used in the treatment of type 2 diabetes, cancer, cardiovascular diseases, neurological and psychiatric disorders, and as a radioprotector. Administrations of Hesperidin can also benefit a variety of cutaneous functions in both normal and diseased skin.
Sources

Hesperidin is abundantly present in citrus fruits (family Rutaceae), including various unripe, sour, and sweet oranges; Citrus aurantium, Citrus sinensis, Citrus unshiu, and Citrus mitis, clementine, lemons, lime, and grapefruits. Hesperidin has also been found to be abundant in the peel and membranous sections of orange peel and various organic citrus items. Citrus rinds contain a large amount of Hesperidin, whereas tangerines peel (Citrus reticulata) may have 5%–10% hesperidin of their dry mass. Hesperidin concentration varies in fruit parts, whereas citrus flavedo, albedo, membrane, and pith contain higher amounts of Hesperidin as compared to other parts (juice vesicles and seeds). About 50–60 mg of Hesperidin is present in 100 mL orange juice, and its amount is five times more in whole fruits as solid parts contain a significant portion of Hesperidin.
Extraction method
Several procedures for extracting flavonoids, including Hesperidin, have been explored, taking into account those that are environmentally friendly. Common extraction methods include dipping, percolation, reflux or continuous reflux. The quality of an extract and the efficiency of a procedure are influenced by several factors, such as solvent type, temperature, extraction time and liquid-solid ratio. Several methodologies based on accelerated solvent extraction (ASE), microwave-assisted extraction (MAE), ultrasound-assisted extraction (USE), subcritical water extraction (SWE), pressurized liquid extraction (PLE), and high hydrostatic pressure (HHP) have been used for isolation of Hesperidin from plant materials.
Two sequential extractions using 90% methanol for 20 min agitation at 55 °C were considered as optimal for the isolation of Hesperidin (24.77 mg/g dw) from the sweet orange pulp (Citrus sinensis L.), while 90% ethanol under the same conditions 17.9 mg/g of Hesperidin was obtained (with significant differences at p < 0.05). Gómez-Mejia et al. proposed extraction from orange peels with a low concentration of ethanol (maximum 40%, v/v) run in an ultrasound bath for 10–15 min. It was stated that a relatively high temperature (90 °C) could be compensated for by a very rapid and efficient extraction process. On the other side, a DMSO: methanol mixture (1:1) turned out to be a better medium during 10 min of ultrasound operation to extract Hesperidin from mandarin (Citrus reticulata Blanco) rinds. The yield of Hesperidin from peels of Citrus unshiu fruits after the MAE process (70% aqueous ethanol, heating 140 °C, 7 min) was comparable to the amount extracted at room temperature for 30 min using the DMSO: methanol (1:1) mixture. The maximum yield of Hesperidin from Citrus unshiu peel using SWE method was obtained at 160 °C for an extraction time of only 10 min. It was 1.9-, 3.2- and 34.2-fold higher than those when 70% ethanol or methanol and hot water, respectively, were used.
References:
[1] PYRZYNSKA K. Hesperidin: A Review on Extraction Methods, Stability and Biological Activities.[J]. ACS Applied Energy Materials, 2022. DOI:10.3390/nu14122387.You may like
Related articles And Qustion
See also
Lastest Price from Hesperidin manufacturers

US $1200.00-1100.00/ton2025-09-30
- CAS:
- 520-26-3
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M
US $1.00/KG2025-09-14
- CAS:
- 520-26-3
- Min. Order:
- 1KG
- Purity:
- 99%+
- Supply Ability:
- 2000 kgs