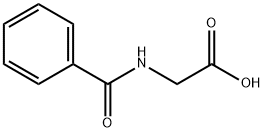
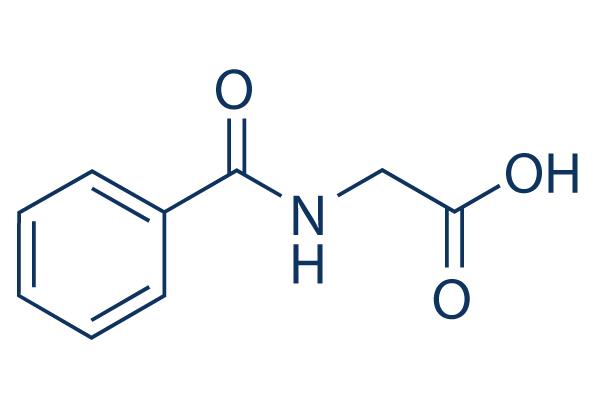
Hippuric acid: Preparation and Biological functions
Introduction
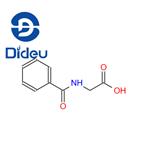
Hippuric acid, which is Benzoylamino-acetic acid, is used for salt formation with Methenamine to yield a 1:1 salt. Even though the therapeutic dose of Methenamine is already high, with a maximum daily dose of Methenamine of 880 mg and a low molar mass of Methenamine of 140 g/mol, hippurate with a high molar mass of 179 g/mol was chosen as salt former, yielding more than 50% (w/w) of salt former in the API. From the highest daily dose, a maximum daily intake of hippurate is calculated as 1120 mg. Neither further hippurate salts have been approved for oral formulations nor intranveneous application.
Preparation
Hippuric acid has been prepared from the urine of herbivorous animals: 1 by heating benzamide with chloroacetic acid;2 by heating benzoic anhydride and glycine;3 by heating benzoic acid and glycine;4 by heating benzoyl chloride with silver glycinate suspended in benzene,5 or with glycine and zinc oxide;6 and by the action of benzoyl chloride upon an alkaline solution of glycine.
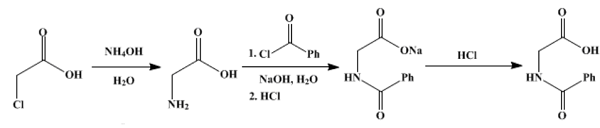
To 3 l (approximately 45 moles) of concentrated ammonium hydroxide (sp. gr. 0.9) in a 5-l round-bottomed flask is added, with shaking, a solution of 95 g (1 mol) of chloroacetic acid in 100 cc of water. The flask is stoppered and allowed to stand for four days. It is then attached to a condenser for distillation, and the solution is concentrated to 600–700 cc. The excess ammonia is recovered during this process by connecting the lower end of the condenser to a wide tube leading to the bottom of a 3-l bottle containing 1.5 l of distilled water and connecting this in the same way with a smaller bottle containing a little water. The bottles are cooled by running water. About 2 l of 20–23 per cent ammonium hydroxide is recovered in the first receiver.
The residual solution is then transferred to a 2-l beaker, a solution of 50 g. (1.25 mol) of sodium hydroxide in 100 cc of water and a little decolorizing carbon are added, and the mixture is boiled until the odor of ammonia is completely absent. The solution is filtered by suction, diluted to 500 cc. with water, and transferred to a 2-l round-bottomed flask equipped with a mechanical stirrer and cooled with running water. While stirring and cooling below 30°, 150 g (1.1 mol) of benzoyl chloride and a cold solution of 80 g (2 mol) of sodium hydroxide in 200 cc of water are admitted separately from separatory funnels at such rates that the solution is always only slightly alkaline. About an hour is required to add the reagents, and the mixture is stirred for half an hour longer. It is then poured into 125 cc of concentrated hydrochloric acid in a 2-l beaker, and after cooling, the precipitate is filtered and dried. The weight at this point is 150–160 g. The solid is placed in a beaker with 300 cc of technical carbon tetrachloride, the beaker is covered with a watch glass, and the mixture is boiled gently for ten minutes. The mixture is then cooled slightly, filtered by gentle suction, and the hippuric acid is washed on the filter with 50 cc of carbon tetrachloride. After drying, it weighs 135–140 g. For final purification, the acid is dissolved in about 2 l of boiling water, filtered through a steam-heated funnel and allowed to crystallize without artificial cooling. It then appears in characteristic white needles melting at 186–187°. The yield is 115–122 g (64–68 % of the theoretical amount based on the chloroacetic acid used). Upon concentrating the mother liquor to 200 cc, a further 6–7 g of slightly brown hippuric acid is obtained.
Biological functions
Hippuric acid is formed by conjugating benzoic acid with glycine, partly by gut bacteria, eliminated actively by renal secretion and found in the urine of healthy individuals. Hippuric acid is influenced by dietary intake and, as such, is elevated with the consumption of phenolic analytes like fruits and juices.
Hippuric acid is already described for a long time as a protein-bound uremic toxin and associated with renal function, as it is secreted via organic anion transporters and partly synthesized by the kidneys, reflecting changes because of kidney damage.
Indirect data reported by Gulyassy and by MacNamara and their colleagues, as well as more direct studies on ultrafiltrate collected in dialyzed patients, demonstrated that hippuric acid interferes with the protein binding of drugs such as phenytoin and theophylline. It also interferes with the tubular transport of organic acids. Hippuric acid (molecular weight, 179 Da) behaves like larger molecules because of its protein binding, which tends to increase during dialysis.
The role of hippuric acid in AKI might be substantial as a result of its interference with drug-protein binding and tubular organic acid excretion. Because most patients with AKI receive many drugs, hippuric acid retention results in the liberation of protein-bound drugs from their binding sites and in an enhanced risk for drug toxicity, which is often difficult to quantify.
Hippuric acid is also associated with other energy-intensive processes and has been linked to cardiovascular disease and the onset of neurological symptoms. Early studies showed hippuric acid inhibits glucose utilization in muscle and kidney cortex tissue. It also interacts with other metabolic processes in the kidney, where it was shown to shift ammonia production from mitochondria to the proximal tubular lumen, influencing processes occurring in metabolic acidosis. These findings indicate that hippuric acid plays a vital role in energy homeostasis.
References
[1] Organic Syntheses Procedure (orgsyn.org) https://orgsyn.org/demo.aspx?prep=cv2p0328
[2] Hippuric Acid - an overview | ScienceDirect Topics https://www.sciencedirect.com/topics/medicine-and-dentistry/hippuric-acid
You may like
Related articles And Qustion
Lastest Price from Hippuric acid manufacturers
US $0.00/kg2025-09-26
- CAS:
- 495-69-2
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kgs

US $0.00/KG2025-04-21
- CAS:
- 495-69-2
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month