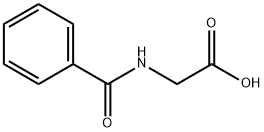
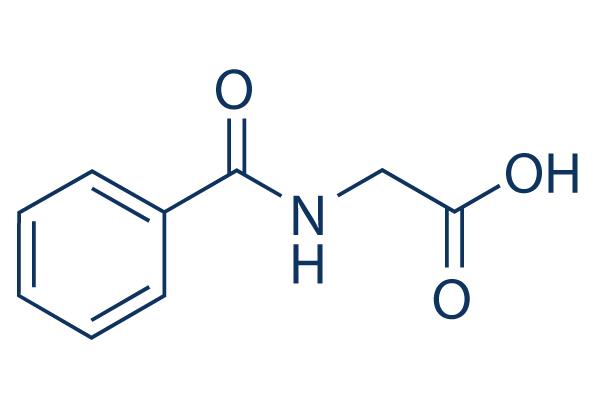
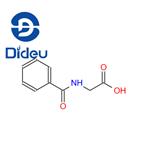
Physiology of Hippuric acid
Microbes resident in the large intestine of the human body help to break down complex aromatic compounds in dietary plant matter (polyphenols), freeing up benzoic acid, which enters the bloodstream. The liver can add the amino acid glycine to benzoic acid to form hippuric acid, which re-enters the blood and is absorbed by the kidneys. As a result, the kidneys excrete hundreds of milligrams of hippuric acid into the urine every day.
Physiology
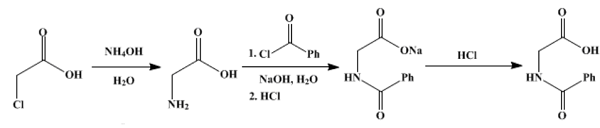
Biochemically, hippuric acid is produced from benzoic acid by direct glycine acylation. It is one of the two conjugates of benzoic acid produced metabolically, the other being the glucuronide. Both are eliminated from the body in urine. Benzoic acid is introduced into the body by ingestion (sodium benzoate is used as a preservative) and by hydrolysis of pharmaceutical agents such as benzyl benzoate.
Hippuric acid may be formed from the essential amino acid phenylalanine through at least two pathways. Phenylalanine undergoes biotransformation to form an alpha-keto acid, phenylpyruvic acid, which can tautomerize to a reactive enol. The benzylic carbon is reactive which undergoes peroxidation followed by the competing pathways to either react with the alpha carbon subsequently form an dioxetanol intermediate followed by formation of oxalic acid and benzaldehyde, or, peroxidation can react with the carboxyl group to form an alpha-keto-beta-peroxylactone intermediate followed by formation of carbon monoxide, carbon dioxide, and benzaldehyde.
Alternatively, under certain conditions, phenylpyruvic acid may undergo a redox mechanism, such as Iron(II) donating an electron, to directly release carbon dioxide, followed by carbon monoxide, for the formation of a stable toluene radical which is resolved by an antioxidant such as ascorbate. In all of the aforementioned cases, benzaldehyde undergoes biotransformation via CYP450 to benzoic acid followed by conjugation to glycine for formation of hippurate which undergoes urinary excretion.Similarly, toluene reacts with CYP450 to form benzaldehyde.
Reactions
Hippuric acid is readily hydrolysed by hot caustic alkalis to benzoic acid and glycine. Nitrous acid converts it into benzoyl glycolic acid, C6H5C(=O)OCH2CO2H. Its ethyl ester reacts with hydrazine to form hippuryl hydrazine, C6H5CONHCH2CONHNH2, which was used by Theodor Curtius for the preparation of hydrazoic acid.
You may like
Related articles And Qustion
Lastest Price from Hippuric acid manufacturers
US $0.00/kg2025-09-26
- CAS:
- 495-69-2
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kgs

US $0.00/KG2025-04-21
- CAS:
- 495-69-2
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month