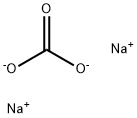
The Electrolytic process of Sodium carbonate
Sodium carbonate and other alkalies
In 1775 the French Academy of Sciences offered an award for a practical method for converting common salt, sodium chloride, into sodium carbonate, a chemical needed in substantial amounts for the manufacture of both soap and glass. Nicolas Leblanc, a surgeon with a bent for practical chemistry, invented such a process. His patron, the duc d’Orléans, set up a factory for the process in 1791, but work was interrupted by the French Revolution. The process was not finally put into industrial operation until 1823 in England, after which it continued to be used to prepare sodium carbonate for almost 100 years.

The Leblanc process
The first step in the Leblanc process was to treat sodium chloride with sulfuric acid. This treatment produced sodium sulfate and hydrogen chloride. The sodium sulfate was then heated with limestone and coal to produce black ash, which contained the desired sodium carbonate, mixed with calcium sulfide and some unreacted coal. Solution of the sodium carbonate in water removed it from the black ash, and the solution was then crystallized. From this operation derives the expression soda ash that is still used for sodium carbonate.
It was soon found that when hydrogen chloride was allowed to escape into the atmosphere, it caused severe damage to vegetation over a wide area. To eliminate the pollution problem, methods to convert the dissolved hydrogen chloride to elemental chlorine were developed. The chlorine, absorbed in lime, was used to make bleaching powder, for which there was a growing demand.
Because calcium sulfide contained in the black ash had a highly unpleasant odour, methods were developed to remove it by recovering the sulfur, thereby providing at least part of the raw material for the sulfuric acid required in the first part of the process. Thus the Leblanc process demonstrated, at the very beginning, the typical ability of the chemical industry to develop new processes and new products, and often in so doing to turn a liability into an asset.
The ammonia-soda (Solvay) process
The Leblanc process was eventually replaced by the ammonia-soda process (called the Solvay process), which was first practiced successfully in Belgium in the 1860s. In this process, sodium chloride as a strong brine is treated with ammonia and carbon dioxide to give sodium bicarbonate and ammonium chloride. The desired sodium carbonate is easily obtained from the bicarbonate by heating. Then, when the ammonium chloride is treated with lime, it gives calcium chloride and ammonia. Thus, the chlorine that was in the original sodium chloride appears as calcium chloride, which is largely discarded (among the few uses for this compound is to melt snow and ice from roads and sidewalks). The ammonia thus regenerated is fed back into the first part of the process. Efficient recovery of nearly all the ammonia is essential to the economic operation of the process, the loss of ammonia in a well-run operation being no more than 0.1 percent of the weight of the product.
Electrolytic process
Later in the 19th century the development of electrical power generation made possible the electrochemical industry. This not clearly identifiable branch of the chemical industry includes a number of applications in which electrolysis, the breaking down of a compound in solution into its elements by means of an electric current, is used to bring about a chemical change. Electrolysis of sodium chloride can lead to chlorine and either sodium hydroxide (if the NaCl was in solution) or metallic sodium (if the NaCl was fused). Sodium hydroxide, an alkali like sodium carbonate, in some cases competes with it for the same applications, and in any case the two are interconvertible by rather simple processes. Sodium chloride can be made into an alkali by either of the two processes, the difference between them being that the ammonia-soda process gives the chlorine in the form of calcium chloride, a compound of small economic value, while the electrolytic processes produce elemental chlorine, which has nearly innumerable uses in the chemical industry, including the manufacture of plastic polyvinyl chloride, the plastic material produced in the largest volume. For this reason the ammonia-soda process, having displaced the Leblanc process, has found itself being displaced, the older ammonia-soda plants continuing to operate very efficiently but no new ammonia-soda plants being built.
Other important processes
The need for sodium carbonate in the manufacture of soap and glass that led to the Leblanc process also led to the creation of the alkali industry and the chlor-alkali industry, another of the historic landmarks of the chemical industry.
You may like
Related articles And Qustion
See also
Lastest Price from Sodium carbonate manufacturers

US $1200.00-1100.00/ton2025-11-07
- CAS:
- 497-19-8
- Min. Order:
- 1ton
- Purity:
- 99%
- Supply Ability:
- 1000T/M

US $1.00/g2025-08-19
- CAS:
- 497-19-8
- Min. Order:
- 1g
- Purity:
- 99%
- Supply Ability:
- 1000kg