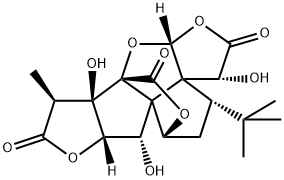
The Chemistry and Biology of Ginkgolide B
General Description
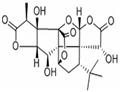
Ginkgolides are complex polyoxygenated diterpenoids isolated from the leaves and root bark of the Ginkgo biloba tree, also known as the maidenhair tree or as the “living fossil”, as its fossils date back to the Jurassic period (170 M years). Ginkgolides B was first isolated by Furukawa in 1932, and its structure was subsequently elucidated in 1967 by Nakanishi.1,2

Figure 1. Properties of Ginkgolide B
Ginkgolides, especially Ginkgolide B, are strong antagonists to the platelet-aggregating receptor (PAFR), which is known as a potent inflammatory factor that plays a role in acute and chronic inflammation. It has been also reported that ginkgolides could serve as effective therapies against central nervous system illnesses such as Alzheimer’s and Parkinson’s disease and multiple sclerosis, and it is also useful in migraine prophylaxis.3
Pharmacological Action of Ginkgolide B
In vitro, Ginkgolide B inhibited human eosinophil and neutrophil locomotion and was significantly more effective in inhibiting chemotaxis. It also inhibited the specific binding of [3H]-PAF to eosinophils and neutrophils. In vivo, intra-abdominal injections of ginkgolide B specially inhibited PAF-induced chemotaxis of polymorphonuclear granulocytes (PMN) and intraepidermal accumulation of inflammatory cells in guinea pigs.4
PAF is an inflammatory mediator capable of inducing protracted inflammation of the airways and bronchial hyperreactivity. Ginkgolide B may have a role in the prevention and treatment of bronchial asthma through inhibiting the decrease of peripheral blood eosinophils and neutrophils. Moreover, ginkgolide B was observed to inhibit activation of human peripheral blood mononuclear cells (PBMC) from asthmatic patients stimulated by phorbol myristate acetate and calcium ionophore and significantly reverse the increase in activation-associated CD45RA expression, with a trend towards decreased expression of HLA-DR. Ginkgolide B combination with cyclosporin A may be useful for preliminary evaluation of novel therapeutic modalities for asthma treatment. In addition, ginkgolide B in combination with the antioxidant carotenoid astaxanthin (ASX) exhibited optimal suppression of T cell activation below fully stimulated values by ginkgolide B, ASX, and their combinations was comparable and for some combinations better than that mediated by two commonly used antihistamines: cetirizine dihydrochloride (CTZ) and azelastine (AZE), which indicated that ASX and ginkgolide B may be another approach to pharmacological treatment of asthma as another novel anti-asthmatic formulations.5
Recent studies conducted with various molecular, cellular, and whole animal models have revealed that ginkgolide B may have anticancer (chemopreventive) properties that are related to their antioxidant, anti-angiogenic and gene-regulatory actions. The peripheral-type benzodiazepine receptor (PBR) expression and localization correlate with human breast cancer cell proliferation and aggressive phenotype expression. Ginkgolide B decreased PBR expression in a time- and dose-dependent manner and cell proliferation in the highly aggressive, rich in PBR, human breast cancer cell line MDA-231, whereas they did not affect the proliferation of the non-aggressive human breast cancer cell line MCF-7, which contains PBR in very low level. These in vitro data were further validated in an in vivo model where ginkgolide B significantly inhibited the nuclear PBR expression and growth of MDA-231 cell xenografts in nude mice.43 Taken together, these data suggest that the manipulation of PBR expression could be used to control tumor growth and that ginkgolide B, under the experimental conditions, exert cytostatic properties.6
Mechanisms Actions of Ginkgolide B
PAF is a potent proinflammatory phospholipid with diverse pathological and physiological effects. The action of PAF is mediated by the PAF receptor, a G protein-coupled membrane-spanning molecule that can engage multiple signaling pathways in various cell types. Inappropriate activation of this signaling pathway is associated with many diseases. Ginkgolides possess strongly specific inhibition to effects of PAF via competitively combining with PAF receptor. Numerous studies demonstrate that Ginkgolide B, as a specific antagonist of PAF, presents beneficial effects on various PAF-related diseases.7
Pharmacokinetics of Ginkgolide B
The maximum concentrations (median)of ginkgolide B in plasma after administration of the maximum daily dose of the different Ginkgo products was 1.38 ng/mL after administration of GeriaforceTM tincture; 4.18 ng/mL after taking Ginkgo fresh plant extract tablets; and 9.99 ng/mL after administration of EGb 761TM tablets.8
Metabolism of Ginkgolide B
Three metabolites were identified in rat urine. One hydroxyl metabolite of Ginkgolide B was identified in rat liver microsomes, and quinidine uncompetitively inhibited the formation of the metabolite; its inhibitor constant (Ki) value for the inhibition of hydroxyl metabolite was estimated to be 8 µmol/L, while α-naphthoflavone, ketoconazole, sulfaphenazole, and diethyldithiocarbamate had no inhibitory effects. Ginkgolide B was metabolized to its hydroxyl metabolite in rats, and CYP2D6 was the major rat CYP isoform responsible for the ginkgolide B metabolism in rat liver microsomes.9
Biosynthesis
Scheme 1. Biosynthesis of ginkgolides through the non-mevalonate pathway. P =PO32-.10
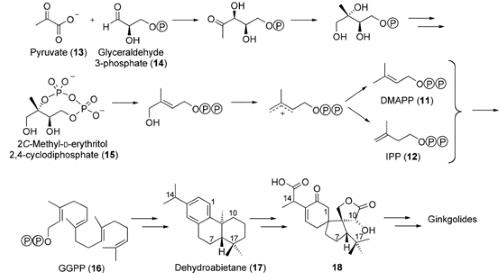
Rohmer used 13C NMR spectroscopy as the major tool to show the existence of non-mevalonate or deoxyxylolose phosphate pathway, in which pyruvate 13 and glyceraldehyde 3-phosphate 14 react to produce 2C-methyl-d-erythritol 2,4-cyclodiphosphate 15, and ultimately DMAPP 11 and IPP 12 (Scheme 1). Independently, Arigoni and Schwarz studied the biosynthesis of ginkgolides with a G. biloba embryo system and 13C-labeled glucose. They showed that ginkgolides were biosynthesized through the non-mevalonate pathway. These comprehensive biosynthetic studies led to the discovery of a novel metabolic pathway for ginkgolides that could be quite widespread. Initially, IPP and DMAPP react to produce the universal diterpene precursor geranylgeranyl pyrophopshate GGPP 16, which is converted into a tricyclic intermediate, levopimaradiene. This leads to dehydroabietane 17, which is transported from the plastids into the cytoplasm. Compound 17 is then converted into the ginkgolides through a complex series of reactions involving several oxidation steps, probably with 18 as an intermediate (Scheme 1).10
Total Synthesis of Ginkgolide B
Scheme 2. Formal Synthesis of Ginkgolides B11
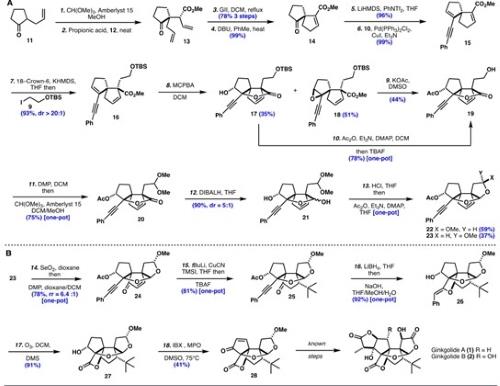
The synthesis commenced by the conversion of ketone 11 to the corresponding dimethyl ketal, which underwent a Claisen rearrangement with allylic alcohol 12 to generate ketone 13. The B-ring was formed via Grubbs II-catalyzed RCM to afford the ring-closed adduct in 78% yield (over three steps), after which, the alkene was conjugated with 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) to afford α,β-unsaturated ester 14 in 99% yield. The ketone was converted to enyne 15 by formation of the corresponding vinyl triflate (96%) followed by a Sonogashira coupling with phenylacetylene 10 (99%). The second quaternary carbon was installed stereoselectively via vinylogous deprotonation of α,β-unsaturated ester 15 with potassium bis(trimethylsilyl)amide (KHMDS)/18-crown-6 followed by the addition of iodoalkane 9 to afford α-alkylated ester 16 in 93% yield (dr >20:1).11
A chemoselective epoxidation with meta-chloroperoxybenzoic acid (MCPBA) on enyne 16 afforded lactone 17 (through an intramolecular cyclization of the α-epoxide) and β-epoxide 18 in 35% and 51% yields, respectively. The lactone 17 was then converted to alcohol 19 in 78% yield via a one-pot process using Ac2O followed by the addition of tetra-nbutylammonium fluoride (TBAF). In parallel, epoxide 18 was treated with KOH in DMSO at 145 °C, which incidentally lactonized onto the methyl ester and deprotected the primary alcohol to afford 19 in a 44% yield. A one-pot oxidation with the Dess-Martin periodinane (DMP) followed by the addition of trimethyl orthoformate and Amberlyst 15 gave the dimethyl acetal 20 in 75% yield. Reduction of the lactone unit with diisobutylaluminum hydride (DIBALH) led to lactol 21 in 90% yield (dr = 5:1), which, after treatment with anhydrous HCl and then with Ac2O, produced anomers 22 and 23 in 96% combined yield (dr = 1.6:1).11
Anomer 23 was converted into the corresponding enone 24 via a one-pot double oxidation with SeO2 and DMP (78% yield, rr = 6.4:1). The stage was set for the stereoselective conjugate addition of the t-Bu group, which required a carefully planned sequence using t-BuLi, CuCN, followed by the addition of TMSI. The resulting trimethylsilyl (TMS) enol ether was then exposed to TBAF, yielding ketone 25 (81%) as a single diastereomer. A stereoselective reduction with LiBH4 provided the corresponding alcohol, which after the addition of NaOH (1 M) underwent a 5-exo-dig cyclization forming the D-ring of 26 in 92% yield. Under these basic conditions the acetate group was hydrolyzed, liberating the alcohol at C3. Ozonolysis of enol ether 26 yielded the hydroxylactone 27 in 91% yield, which was directly oxidized with 2-iodoxybenzoic acid (IBX) and 4-methoxypyridine N-oxide (MPO), leading to enone 28 in 41% yield, which was converted into Ginkgolide B (2) in known six steps.11
References
1.Furukawa, S. Studies on the Constituents of Ginkgo Biloba L. Leaves Part I and II. Sci. Pap. Inst. Phys. Chem. Res. 1932, 19, 27?42.
2.(a) Maruyama, M.; Terahara, A.; Itagaki, Y.; Nakanishi, K. The Ginkgolides. I. Isolation and Characterization of the Various Groups. Tetrahedron Lett. 1967, 8, 299?302. (b) Maruyama, M.; Terahara, A.; Itagaki, Y.; Nakanishi, K. The Ginkgolides. II. Derivation of Partial Structures. Tetrahedron Lett. 1967, 8, 303?308. (c) Maruyama, M.; Terahara, A.; Nakadaira, Y.; Woods, M. C.; Nakanishi, K. The Ginkgolides. III. The Structures of the Ginkgolides. Tetrahedron Lett. 1967, 8, 309?313. (d) Maruyama, M.; Terahara, A.; Nakadaira, Y.; Woods, M. C.; Takagi, Y.; Nakanishi, K. The Ginkgolides. IV. Stereochemistry of the Ginkgolides. Tetrahedron Lett. 1967, 8, 315?319. (e) Woods, M. C.; Miura, I.; Nakadaira, Y.; Terahara, A.; Maruyama, M.; Nakanishi, K. The Ginkgolides. V. Some Aspects of their NMR Spectra. Tetrahedron Lett. 1967, 8, 321?326.
3.Liu, Y.; Shields, L. B. E.; Gao, Z.; Wang, Y.; Zhang, Y. P.; Chu, T.; Zhu, Q.; Shields, C. B.; Cai, J. Current Understanding of PlateletActivating Factor Signaling in Central Nervous System Diseases. Mol. Neurobiol. 2017, 54, 5563?5572.
4.Kurihara,K, Wardlaw, AJ, Moqbel, R, Kay, AB. Inhibition of platelet-activating factor (PAF)-induced chemotaxis and PAF binding to human eosinophils and neutrophils by the specific ginkgolide-derived PAF antagonist, BN52021. J Allergy Clin Immunol 1989; 83: 83-90.
5.Hsieh, KH. Effects of PAF antagonist, BN52021, on the PAF-, methacholine-, and allergen-induced bronchoconstriction in asthmatic children. Chest 1991; 99: 877-882.
6.Papadopoulos, V, Kapsis, A, Li, H, Amri, H, Hardwick, M, Culty, M, et al. Drug-induced inhibition of the peripheral-type benzodiazepine receptor expression and cell proliferation in human breast cancer cells. Anticancer Res 2000; 20: 2835-2847.
7.Kuribara H, Weintraub ST, Yoshihama T, Maruyama Y. An anxiolytic-like effect of Ginkgo biloba extract and its constituent, ginkgolide-A, in mice. J Natural Products 2003; 66: 1333-1337.
8.Woelkart, K., Feizlmayr, E., Dittrich, P., Beubler, E., Pinl, F., Suter, A., & Bauer, R. (2010). Pharmacokinetics of bilobalide, ginkgolide A and B after administration of three different Ginkgo biloba L. preparations in humans. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives, 24(3), 445-450.
9.Wang, D. L., Liang, Y., Chen, W. D., Xie, L., Wang, G. J., & Liu, X. D. (2008). Identification of ginkgolide B metabolites in urine and rat liver cytochrome P450 enzymes responsible for their formation in vitro. Acta Pharmacologica Sinica, 29(3), 376-384.
10.Str?mgaard, K., & Nakanishi, K. (2004). Chemistry and biology of terpene trilactones from Ginkgo biloba. Angewandte Chemie International Edition, 43(13), 1640-1658.
11.Hébert, M., Bellavance, G., & Barriault, L. (2022). Total Synthesis of Ginkgolide C and Formal Syntheses of Ginkgolides A and B. Journal of the American Chemical Society, 144(39), 17792-17796.
You may like
Related articles And Qustion
See also
Lastest Price from Ginkgolide B manufacturers

US $0.00/kg2024-04-12
- CAS:
- 15291-77-7
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 2000ton
US $0.00/mg2023-02-24
- CAS:
- 15291-77-7
- Min. Order:
- 5mg
- Purity:
- ≥98%(HPLC)
- Supply Ability:
- 10 g