MRTX-1719: Bioactivity and Chemical and studies
General description
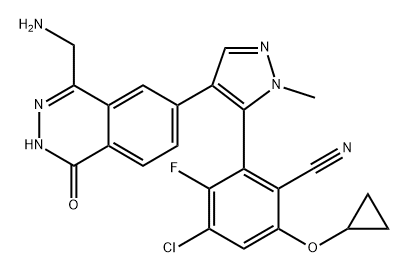
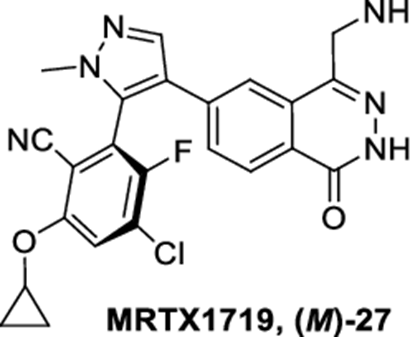
The MRTX-1719, with the CAS No: 2630904-45-7, is known as Benzonitrile, 2-[4-[4-(aminomethyl)-1,2-dihydro-1-oxo-6-phthalazinyl]-1-methyl-1H-pyrazol-5-yl]-4-chloro-6-(cyclopropyloxy)-3-fluoro-,(2R)-, and also named (2R)-2-[4-[4-(aminomethyl)-1,2-dihydro-1-oxo-6-phthalazinyl]-1-methyl-1H-pyrazol-5-yl]-4-chloro-6-(cyclopropyloxy)-3-fluorobenzonitrile. This chemical’s molecular formula is C23H18ClFN6O2 and molecular weight is 464.89. MRTX1719 is a potent and selective binder to the PRMT5MTA complex and selectively inhibits PRMT5 activity in MTAP -deleted cells compared to MTAP-wild-type cells.[1] MRTX1719 is a class 3 atropisomeric compound that requires a chiral synthesis or a chiral separation step in its preparation. PRMT5 forms a complex with MEP50 (methylosome protein 50), which is required for substrate recoginition and orientation and is also required for PRMT5-catalyzed histone 2A and histone 4 methyltransferase activity. Homozygous deletions of p16/CDKN2a are prevalent in cancer and these mutations commonly involve the co-deletion of adjacent genes, including the gene encoding methylthioadenosine phosphorylase.It is estimated that approximately 15% of all human cancers have a homozygous deletion of the MTAP gene. Cells lacking MTAP activity have elevated levels of the MTAP substrate, methylthioadenosine (MTA), which is a potent inhibitor of PRMT5. Inhibition of PRMT5 activity results in reduced methylation activity and increased sensitivity of cellular proliferation to PRMT5 depletion or loss of activity. Hence, the loss of MTAP activity reduces methylation activity of PRMT5 making the cells selectively dependent on PRMT5 activity. MTA-cooperative inhibition of PRMT5 activity in MTAP deleted. cancers will provide therapeutic benefit for a wide range of cancers. The invention of MRTX-1719 provide this therapeutic benefit as MTA-cooperative inhibitors of PRMT5 that negatively modulate the activity of MTA-bound PRMT5 in a cell, particularly an MTAP-deficient cell, or for trating various forms of MTAP-associated cancer. There is a need to develop new MTA-cooperative PRMT5 inhibitors that are capable of inhibiting PRMT5 activity in the presence of elevated MTA concentrations, particularly in MTAP-deficient cells.[1]
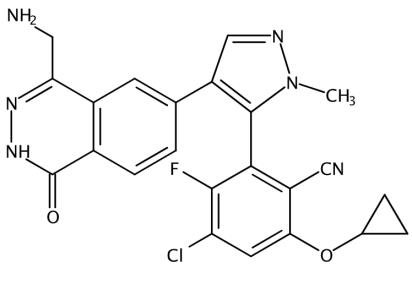
Figure 1 the molecular formula of MRTX-1719
Indication
MRTX1719 is an inhibitor of the PRMT5/MTA complex and recently entered clinical trials for the treatment of MTAP-deleted cancers, it is a class 3 atropisomeric compound that requires a chiral synthesis or a chiral separation step in its preparation. PRMT5 forms a complex with MEP50 (methylosome protein 50), which is required for substrate recoginition and orientation and is also required for PRMT5-catalyzed histone 2A and histone 4 methyltransferase activity. Homozygous deletions of p16/CDKN2a are prevalent in cancer and these mutations commonly involve the co-deletion of adjacent genes, including the gene encoding methylthioadenosine phosphorylase (MT\P) It is estimated that approximately 15% of all human cancers have a homozygous deletion ofthe MTAP gene. Cells lacking MTAP activity have elevated levels of the MTAP substrate, methylthioadenosine (MTA), which is a potent inhibitor of PRMT5. Inhibition of PRMT5 activity results in reduced methylation activity and increased sensitivity of cellular proliferation to PRMT5 depletion or loss of activity. Hence, the loss of MTAP activity reduces methylation activity of PRMT5 making the cells selectively dependent on PRMT5 activity. MTA-cooperative inhibition of PRMT5 activity in MTAP deleted. Cancers will provide therapeutic benefit for a wide range of cancers. The invention of MRTX-1719 provide this therapeutic benefit as MTA-cooperative inhibitors of PRMT5 that negatively modulate the activity of MTA-bound PRMT5 in a cell, particularly an MTAP-deficient cell, or for treating various forms of MTAP-associated cancer. There is a need to develop new MTA-cooperative PRMT5 inhibitors that are capable of inhibiting PRMT5 activity in the presence of elevated MTA concentrations, particularly in MTAP-deficient cells.[2]
Pharmacodynamics
Daily oral administration of MRTX1719 to tumor xenograft-bearing mice demonstrated dose-dependent inhibition of PRMT5-dependent symmetric dimethylarginine protein modification in MTAP-deleted tumors that correlated with antitumor activity. A 4-(aminomethyl)phthalazin-1(2H)-one hit was identified through a fragment-based screen, followed by X-ray crystallography, to confirm binding to the PRMT5.bul. MTA complex. Fragment growth supported by structural insights from X-ray crystallography coupled with optimization of pharmacokinetic properties aided the discovery of development candidate MRTX1719.[1]
Preparation
To a solution of Intermediate HB (408mg, 1.14mmol, LOOeq.) and 1-[[bromo(difluoro)methyl]-ethoxy-phosphoryl]oxyethane (306mg, 1.14mmol, LOOeq.) in acetonitrile (8.00mL) was added to potassium fluoride (133mg, 2.29mmol, 53.6pL, 2.00eq.) at 20°C. The mixture was stirred at 30°C for 16 hours. The reaction mixture was concentrated under reduced pressure and the residue was purified by column chromatography (silicon dioxide, (Ethyl acetate/Petroleum ether 10-35%) to give a mixture of 2-(4-bromo-1-(difluoromethyl)-17/-pyrazol-5-yl)-4-chloro-6-cyclopropoxy-3-fluorobenzonitrile and 2-(4-bromo-l-(difluoromethyl)-l//-pyrazol-3-yl)-4-chloro-6-cyclopropoxy-3-fluorobenzonitrile (300mg, 65%yield). The mixture was further separated by SFC (column: Phenomenex-Cellulose-2(250mm*30mm, 10pm); mobile phase:[0.1%NH3x H2OMeOH]; B%: 5%-5%,3;80min) to give 2-(4-bromo-l-(difluoromethyl)-l/7-pyrazol-5-yl)-4-chloro-6-cyclopropoxy-3-fluorobenzonitrile (70.0 mg, 23.3% yield) as a white solid. To a solution of 2-(4-bromo-l-(difluoromethyl)-l/7-pyrazol-5-yl)-4-chloro-6-cyclopropoxy-3-fluorobenzonitrile (70.0mg, 172pmol, 1.00eq.) and Intermediate J (62.2mg, 155pmol, 0.90eq.) in dioxane (2.0mL) and water (0.40mL) was added to sodium bicarbonate (43.4mg, 57pmol, 3.00eq.) and (Ad2n-BuP)-Pd G3 (12.5mg, 17.2pmol, 0.10eq.) at 15°C. The mixture was stirred at 80°C for 16 hours under nitrogen. The mixture was diluted with water (5.0mL) and extracted with ethyl acetate (5.0mL x 3). The organic layer was dried over sodium sulfate and then concentrated under vacuum to get the residue. The residue was purified by prep-HPLC (column:Phenomenex luna C18 150*25mm* 10pm; mobile phase: [water (0.1%TFA)-ACN]; B%: 45%-75%,l1.5min) to give Zert-butyl((7-(5-(3-chloro-6-cyano-5-cyclopropoxy-2-fluorophenyl)-l-(difluoromethyl)-17/-pyrazol-4-yl)-4-oxo-3,4-dihydrophthalazin-l-yl)methyl)carbamate (30.0 mg, 23.3% yield) as a white solid. The tert-butyl((7-(5-(3-chloro-6-cyano-5-cyclopropoxy-2-fluorophenyl)-l-(difluoromethyl)-1H-pyrazol-4-yl)-4-oxo-3,4-dihydrophthalazin-l-yl)methyl)carbamate (30.0mg, 50.0pmol, 1.00eq.) was separated by SFC (column: REGIS (R,R)WHELK01 (250mm*25mm, 10um); mobile phase: [0.1%NH3 χ H2O IPA];B%: 35%-35%,4;35min) to afford (M)-ter/-butyl((7-(5-(3-chloro-6-cyano-5-cyclopropoxy-2-fluorophenyl)-l-(difluoromethyl)-l/7-pyrazol-4-yl)-4-oxo-3,4-dihydrophthalazin-l-yl)methyl)carbamate(13.0mg, 28.3% yield) as a yellow solid and (P)-tert-butyl((7-(5-(3-chloro-6-cyano-5-cyclopropoxy-2-fluorophenyl)-l-(difluoromethyl)-l.H-pyrazol-4-yl)-4-oxo-3,4-dihydrophthalazin-l-yl)methyl)carbamate (13.0mg, 40.7% yield) as a yellow solid. (P)-tert-butyl((7-(5-(3-chloro-6-cyano-5-cyclopropoxy-2-fluorophenyl)-l-(difluoromethyl)-l/7-pyrazol-4-yl)-4-oxo~3,4-dihydrophthalazin-l-yl)methyl)carbamate(10.0mg, 16.6pmol, 1.00eq.) in DCM (0.50mL) was added to TFA (385mg, 3.38mmol, 0.25mL, 203 eq.) at 20°C and stirred for 0.5 hour. The reaction mixture was concentrated under reduced pressure and the residue was purified by prep-HPLC (column: Welch Xtimate C18 150x25mmx5um; mobile phase: [water (0.05%HCl)-ACN]; B%: 20%-50%, 1 Omin) to give (P)-2-(4-(4-(aminomethyl)-1-oxo-1,2-dihydrophthalazin-6-yl)-1-(difluoromethyl)-l/f-pyrazol-5-yl)-4-chloro-6-cyclopropoxy-3-fluorobenzonitrile(3.66mg, 39.3%yield) as off-white solid. To a solution of(^-tert-butyl((7-(5-(3-chloro-6-cyano-5-cyclopropoxy-2-fluorophenyl)-1-(difluoromethyl)-1/f-pyrazol-4-yl)-4-oxo3,4-dihydrophthalazin-l-yl)methyl)carbamate (10.0mg, 16.6pmol, 1.00eq) in DCM (0.50mL) was added to TFA (385mg, 3.38mmol, 0.25mL, 203eq) at 20°C and stirred for 0.5 hour. The reaction mixture was concentrated under reduced pressure and the residue was purified by prep HPLC (column:Welch Xtimate C18 150x25mmx5um; mobile phase: [water (0.05%HCl)-ACN]; B%: 20%-50%, l0min) to give MRTX-1719 (5.06mg, 55.82% yield) as a white solid.[3]
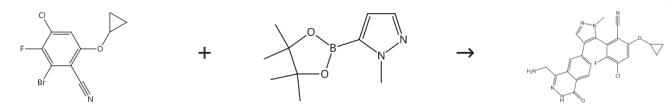
Figure 2 Preparation of MRTX17
References
1.Smith C R, Aranda R, Bobinski T P, et al. Fragment-Based Discovery of MRTX1719, a Synthetic Lethal Inhibitor of the PRMT5MTA Complex for the Treatment of MTAP -Deleted Cancers[J]. 2022.
2.Smith C R, Kulyk S, et al. Design and evaluation of achiral, non-atropisomeric 4-(aminomethyl)phthalazin-1(2H)-one derivatives as novel PRMT5/MTA inhibitors[J].Bioorganic medicinal chemistry, 2022,71,116947.
3.Engstrom, Lars Daniel; Olson, Peter; Christensen, James Gail. Combination therapies using PRMT5 inhibitors for the treatment of cancer[P]. 2022,WO2022216648,A120221013.