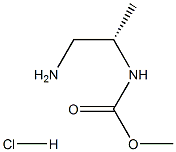
The introduction of (S)-Methyl 1-aMinopropan-2-ylcarbaMate hydrochloride
General description
The(S)-Methyl 1-aMinopropan-2-ylcarbaMate hydrochloride, with the CAS No: 1229025-32-4, is also known as N-[(1S)-2-Amino-1-methylethyl]-carbamic acid methyl ester hydrochloride (1:1). This chemical’s molecular formula is C5H13ClN2O2 and molecular weight is 168.62. The (S)-Methyl 1-aMinopropan-2-ylcarbaMate hydrochloride is a kind of synthetic intermediate. Its structure is as follows:
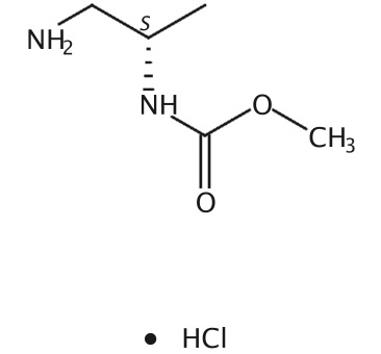
Figure 1 Structure of (S)-Methyl 1-aMinopropan-2-ylcarbaMate hydrochloride.
Chemical synthesis of (S)-Methyl 1-aMinopropan-2-ylcarbaMate hydrochloride
The (S)-Methyl 1-aMinopropan-2-ylcarbaMate hydrochloride can be synthesized according to the previous work [1]. To a suspension of (S)-1,2-diaminopropane dihydrochloride (50 g, 340 mmol) in dichloromethane (500 mL) was added potassium carbonate (1190 mmol). The suspension was stirred and filtered to collect the filtrate. The filtrate was cooled to 0-5° C. and stirred while benzyl chloroformate (51 ml, 357 mmol) was added dropwise. Following completion of the addition, the reaction mixture was stirred for 3 h at 0-5° C., and was then allowed to warm to rt and was stirred at rt overnight. To this mixture was added dropwise triethylamine (71 ml, 510 mmol) and the mixture was cooled to 0-5° C. Methyl chloroformate (28 ml, 357 mmol) was added slowly at 0-5° C. and the mixture was allowed to warm to rt and was stirred overnight. The mixture was poured into water. The organic volatiles were removed under vacuum. The resulting aqueous slurry was filtered to collect the solids, and the filter cake was then washed with ethyl acetate to provide a white solid (65 g, 92-94% HPLC purity). Multiple recrystalizations from ethyl acetate provided the title compound as a white solid, HPLC purity 99.5%. A solution of (S)-Benzyl 2-(methoxycarbonylamino)propylcarbamate in methanol was hydrogenated over a 5% palladium/C catalyst at 50-60 psi. The reaction mixture was filtered and the filtrate was concentrated under vacuum to give a colorless oil. 60 g of the colorless oil was dissolved in 200 mL of anhydrous dichloromethane and the solution was cooled to 0-5 °C in an ice-water bath. A solution of HCl in methanol (ca. 75 mL) was added dropwise until the pH of the solution was <1. The resulting suspension was stirred at 0-5 °C for 30 min, then the solid was collected via filtration. The solid was washed with dichloromethane and then with hexanes to give the title compound as a white solid. Furthermore, a simple synthesis method with single step is as follows [1]: A solution of (S)-Benzyl 2-(methoxycarbonylamino)propylcarbamate in methanol was hydrogenated over a 5% palladium/C catalyst at 50-60 psi. The reaction mixture was filtered and the filtrate was concentrated under vacuum to give a colorless oil. 60 g of the colorless oil was dissolved in 200 mL of anhydrous dichloromethane and the solution was cooled to 0-5 °C in an ice-water bath. A solution of HCl in methanol (ca. 75 mL) was added dropwise until the pH of the solution was <1. The resulting suspension was stirred at 0-5 °C for 30 min, then the solid was collected via filtration. The solid was washed with dichloromethane and then with hexanes to give the title compound as a white solid.
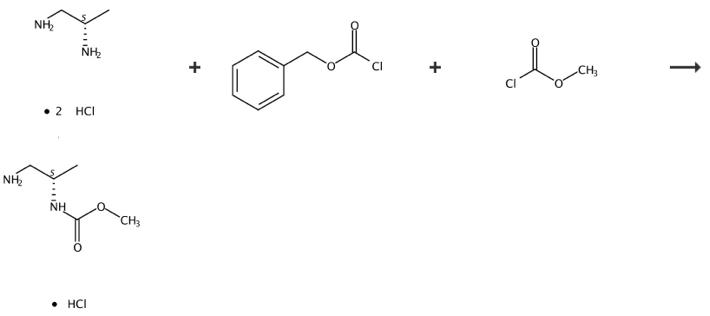
Figure 2 Chemical synthesis of (S)-Methyl 1-aMinopropan-2-ylcarbaMate hydrochloride.
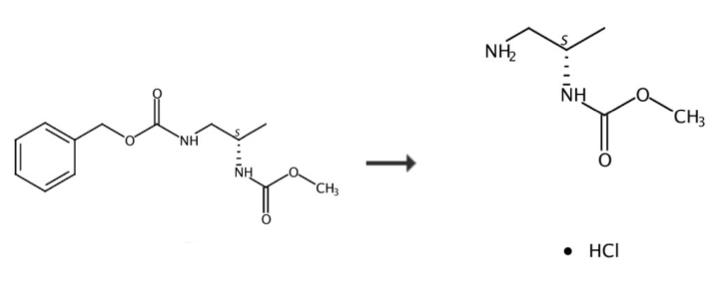
Figure 3 Chemical synthesis with one step of (S)-Methyl 1-aMinopropan-2-ylcarbaMate hydrochloride.
Analytical method of (S)-Methyl 1-aMinopropan-2-ylcarbaMate hydrochloride
(S)-Methyl 1-aMinopropan-2-ylcarbaMate hydrochloride can be analyzed by GC method [2]. Briefly, weigh 0.02 g (accurate to 0.00002 g) of (S)-Methyl 1-aMinopropan-2-ylcarbaMate hydrochloride into a 50 mL volumetric flask, add acetonitrile to dissolve it, and shake it up after constant volume. Accurately transfer 1.0 mL of the above solution into a 10 mL volumetric flask, and fix the volume with acetonitrile, shake it up, and use it for standby. Gas chromatograph (Agilent7820): equipped with FID hydrogen flame ionization detector, chromatographic column: DM-624 (30 mm × 0.53 mm,3 μ m). Temperature: vaporization chamber 200 ℃, detector 250 ℃; Column temperature: programmed temperature rise: the initial temperature is 50 ℃, keep it for 1 min, increase it to 120 ℃ at the rate of 10 ℃/min, keep it for 3 min, and then increase it to 250 ℃ at the rate of 12 ℃/min; Gas flow (mL/min): carrier gas (N2) 2.0, compensation gas (N2) 25, hydrogen 30, air 300; Injection volume: 1 μ L;Retention time: about 5.87 min for (S)-Methyl 1-aMinopropan-2-ylcarbaMate hydrochloride.
Toxicity of (S)-Methyl 1-aMinopropan-2-ylcarbaMate hydrochloride
As a potential genotoxic impurity, (S)-Methyl 1-aMinopropan-2-ylcarbaMate hydrochloride and its analogues have been proven to induce benign or malignant tumors, especially lung cancer and liver cancer, as early as the 1960s and 1970s [3].
References
[1] Huang, S et al. Preparation of sulfonamidophenylimidazolylpyrimidine derivatives and analogs for use as protein kinase inhibitors. PCT Int. Appl., 2011025927, 03 Mar 2011.
[2]Park, SK, et al. Analysis of ethyl carbamate in koreansoy sauce using high-performance liquid chromatography withflour-rescence detection or tandem mass spectrometry and gas chromatographywith mass spectrometry. Food Control, 2007, 18(8): 975-982.
[3]Sakano, K, et al. Metabolism of carcino-genicurethane to nitric oxide is involved in oxidative DNA damage. Free Radical Biol Med, 2002, 33(5): 703-714.