The Chemical Name, Properties and Uses of H2CO3
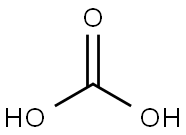
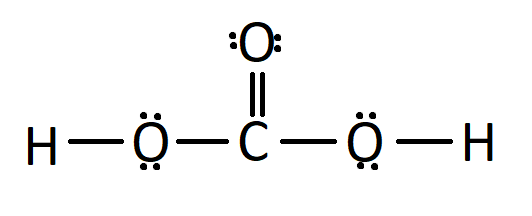
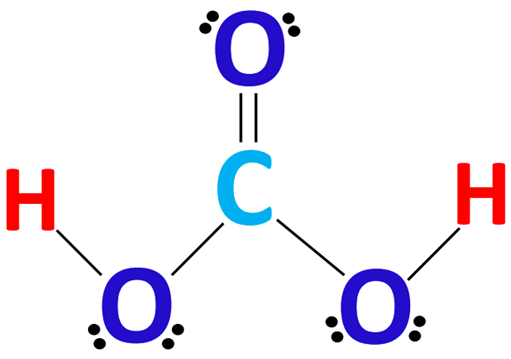
Carbonic acid, with the chemical formula H2CO3, is a carbon-containing compound often found in solutions of carbon dioxide in water. It can also be represented as OC(OH)2 due to the presence of a carbon-oxygen double bond. This article will collate the chemical name, uses and properties of H2CO3.

Chemical Name
Referred to as "respiratory acid" or "volatile acid," carbonic acid is unique for being the sole acid exhaled in gaseous form by human lungs. It is a weak acid, capable of forming carbonate and bicarbonate salts.
One significant effect of carbonic acid is its ability to dissolve limestone, leading to the creation of calcium bicarbonate (Ca(HCO3)2) and various limestone formations like stalagmites and stalactites.
Properties
Below are outlined some key physical and chemical attributes of carbonic acid:
Physical Properties
Molar Mass: 62.024 grams per mole
Density (standard state): 1.668 grams per cubic centimeter
pKa Value: 6.35
Conjugate Base: Bicarbonate
State: Primarily exists in solution, although solid samples have been prepared by NASA scientists.
Chemical Properties
Weakness: H2CO3 is an unstable weak acid.
Dissociation: Partial dissociation in water produces H+ and HCO3– ions.
Diprotic Nature: Can form bicarbonates and carbonates.
Salts Formation: Bicarbonate salts result from small base quantities, while excess base yields carbonate salts.
Uses
This compound finds diverse applications:
- Preparation of carbonated beverages like sparkling water and wine.
- Use in precipitating ammonium salts such as ammonium persulfate.
- Facilitation of carbon dioxide transportation out of the body.
- Protonation of nitrogen-containing bases in blood serum.
- Treatment of ringworm and dermatitis.
- Effective cleaning agent for contact lenses.
- Oral consumption for inducing vomiting in cases of drug overdose.