ChemicalBook > Articles Catagory List >Inorganic-chemistry >in-clo3-what-is-the-oxidation-state-of-chlorine
In ClO3-, what is the oxidation state of chlorine?
Feb 27,2024
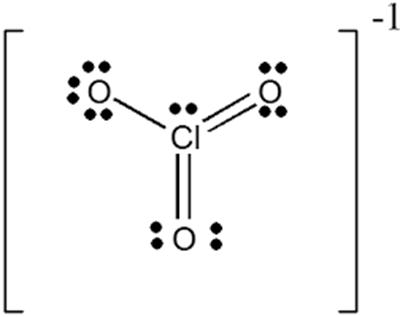
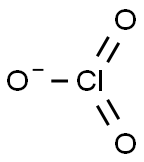
The oxidation state of chlorine is +5 in ClO3-. The Cl forms double bonds with two of the oxygen molecules and a single bond with the third. This leaves the third oxygen negatively charged with a single unpaired electron. The oxidation state of chlorine in ClO3- is the sum of the oxidation number provided by the anions (-2 for each oxygen), less the balance charge on the compound (-1 for ClO3-). Thus, the chlorine oxidation state is -2x3 - (-1) = +5 (negative charges must equal positive charge).