How to Determine the Ionic Charge of Carbonic acid?
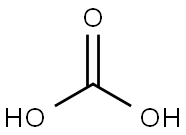
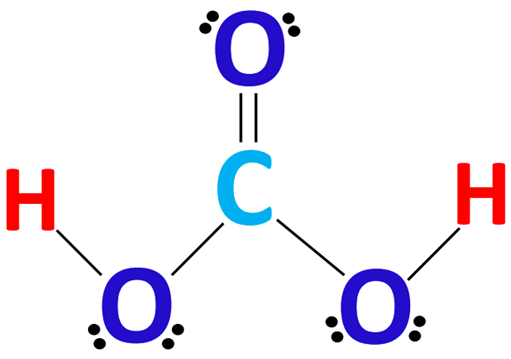
Carbonic acid is a carbon-containing compound which has the chemical formula H2CO3. Solutions of carbon dioxide in water contain small amounts of this compound. Its chemical formula can also be written as OC(OH)2 since there exists one carbon-oxygen double bond in this compound. Carbonic acid is a neutral molecule. However, carbonic acid is unstable and often dissociates into a proton (which is donated to a base) and bicarbonate, which has a charge of -1. Loss of the other proton yields carbonate, which has a charge of -2.
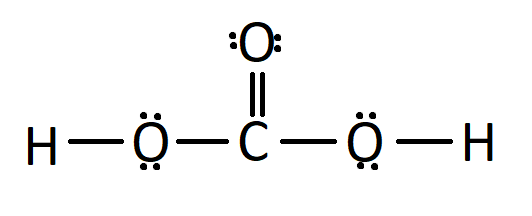
Carbonic acid is often described as a respiratory acid since it is the only acid that is exhaled in the gaseous state by the human lungs. It is a weak acid and it forms carbonate and bicarbonate salts. H2CO3 can dissolve limestone, which leads to the formation of calcium bicarbonate (Ca(HCO3)2. This is the reason for many features of limestone, such as stalagmites and stalactites.
Physical Properties
The molar mass of carbonic acid is 62.024 grams per mole.
Its density in its standard state is 1.668 grams per cubic centimetre.
The compound H2CO3 has a pKa value of 6.35.
The conjugate base corresponding to carbonic acid is the bicarbonate.
This compound generally exists as a solution. However, it has been reported that solid H2CO3 samples have been prepared by NASA scientists.
Chemical Properties
H2CO3 is a weak acid and is unstable in nature.
It undergoes partial dissociation in the presence of water to yield H+ and HCO3– (bicarbonate) ions.
Carbonic acid is a diprotic acid, and can hence form two types of salts, namely bicarbonates and carbonates.
Addition of a small quantity of a base to H2CO3 yields bicarbonate salts whereas addition of a base in excess yields carbonate salts.
It can be noted that carbonic acid can be obtained as a by-product of industrial fermentation processes or the burning of fossil fuels at an industrial scale.
Uses of Carbonic Acid
H2CO3 is a very important compound with a wide range of applications. Some of these uses of carbonic acid are listed below.
The preparation of carbonated water, sparkling wine, and other aerated drinks involve the use of carbonic acid.
H2CO3 is used in the precipitation of many ammonium salts such as ammonium persulfate.
It helps in the transportation of carbon dioxide out of the body.
Various bases containing nitrogen in blood serum are protonated by H2CO3
Ringworm and other dermatitides are treated via the application of carbonic acid over the affected area.
Solutions containing this compound are very effective in the cleaning of contact lenses.
It can be consumed orally in order to induce vomiting whenever required (such as in drug overdose cases).