The application of L-Ergothioneine
General description
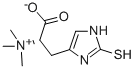
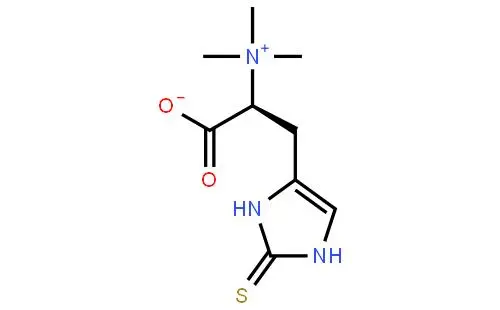
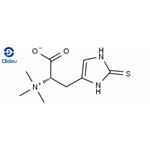
L-Ergothioneine (EGT) is a L-histidine derivative that is N(alpha), N(alpha),N(alpha)-trimethyl-L-histidine in which the hydrogen at position 2 on the imdazole ring is replaced by a mercapto group. A naturally occurring metabolite of histidine synthesized by bacteria and fungi with antioxidant properties. It is found ubiquitously in plants and animals and is present in many human foodstuffs. It has a role as an antioxidant, a fungal metabolite, a plant metabolite, a xenobiotic metabolite and a chelator.
It is an amino-acid betaine, a L-histidine derivative and a sulfur-containing amino acid. It is a conjugate base of an ergothioneine(1+). It is a tautomer of an ergothioneine thione form. It has intrigued scientists subsequent to its discovery in 1909 by the French pharmacist Charles Tanret. Over the years, attempts have been made to characterize the origin, fate and function of this unique compound. Initially discovered in a fungal contaminant of rye grain, EGT is present in some anaerobic and aerobic micro-organisms, as well as plants, and was later found to be highly mconcentrated in the cells, organs and tissues of most animals and humans.
Application and Pharmacology
L-Ergothioneine (EGT), a thiourea derivative of histidine, is a naturally occurring amino acid, and it has received much attention as a food preservative in the food industry because of its strong antioxidant effects. It is a highly stable antioxidant that is able to neutralize free radicals and protect cells from oxidative stress, which is a major contributor to various health problems, including aging, cancer, and heart disease. Ergothioneine has also been shown to have anti-inflammatory properties, which may further contribute to its potential health benefits.
The avidity by which dietary EGT is assimilated by bodily tissues and its participation in several important cellular activities such as redox homeostasis or energy regulation jointly suggest a crucial physiological role for this natural molecule. It is well established that EGT acts as a cell-protective molecule under oxidative stress or inflammatory conditions. Even more interesting is the idea that entry of EGT into the cells would be regulated by the inducible expression of its specific transporter OCTN1/ETT [13], which is dependent on the oxidative stress level. Early attempts to determine whether EGT would function as a vitamin were abandoned because of the lack of a well-defined animal model of EGT deficiency. However, such a physiological role has been suggested recently. EGT has mainly been viewed as an antioxidant or cytoprotective agent against oxidative stress, as well as an anti-inflammatory agent both in vitro and in different animal models[1]. Ergothioneine is found in various foods, including mushrooms, beans, and oat bran. It can also be obtained through dietary supplements, which are available in the form of capsules or tablets.
1. L‑Ergothioneine Exhibits Protective Effects against Dextran Sulfate Sodium-Induced Colitis in Mice[2]. L-Ergothioneine (EGT) widely existing in mushrooms has various physiological activities. EGT administration, especially at the high dose level, prevented the body weight loss, the colon shortening, and the increase in disease activity index and spleen index caused by dextran sulfate sodium (DSS). Moreover, EGT supplementation attenuated DSS-induced gut barrier damage by enhancing the expression of tight-junction protein and recovering the loss of gut mucus layer. Furthermore, EGT considerably decreased the colonic myeloperoxidase (MPO) activity induced by DSS, but no significant differences were observed in the concentrations of IL-6 and TNF-α in colon tissues. Additionally, EGT downregulated the populations of CD4+ T cells and macrophages, indicating that EGT stabilized the immune response caused by DSS. EGT can alleviate DSS-induced colitis and provide important insights concerning the potential anticolitis activity of such food products. (Figure 1)
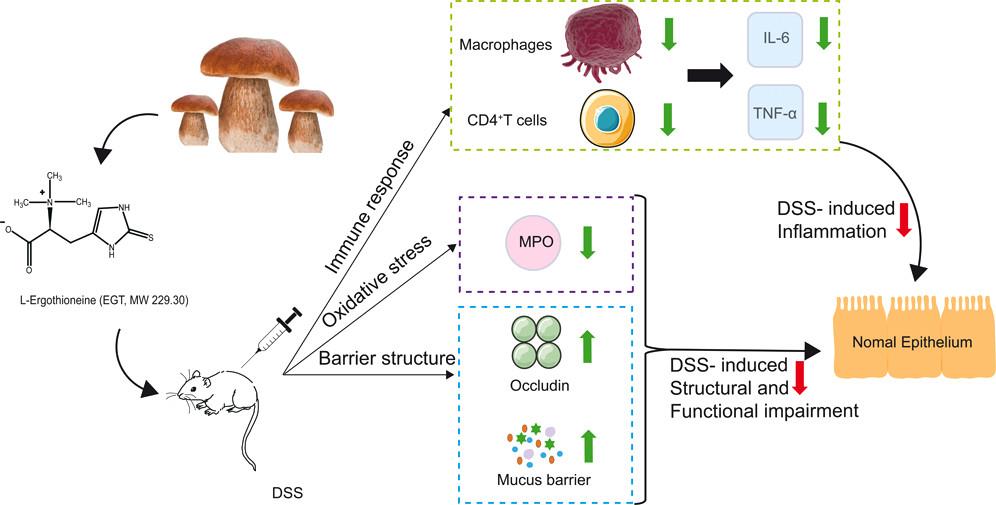
Figure 1 L‑Ergothioneine Exhibits Protective Effects against Dextran Sulfate Sodium-Induced Colitis in Mice
2. Ergothioneine as a natural antioxidant against oxidative stress-related diseases. EGT is found to be highly accumulated in tissues that are susceptible to oxidative damage, and it has attracted extensive attention due to its powerful antioxidant activity and the tight relationships of this natural product with various oxidative stress-related diseases. More and more in vitro and in vivo experiments proved that the antioxidant function of EGT was superior to some other natural antioxidants. As a non-toxic natural antioxidant, the antioxidant function of EGT makes it have the huge therapeutic or preventive potential for many oxidative stress-mediated diseases. Therefore, synthetic EGT has gradually attracted people’s attention and has been widely used in the food and cosmetics industry[3].
Synthesis
Natural EGT-producing strains and their EGT synthetic pathways have been identified for industrial EGT synthesis. EGT was initially discovered in the ergot fungus and subsequently identified in several organisms, such as Schizosaccharomyces pombe (fission yeast), Neurosporacrassa (filamentous fungus), and Mycobacterium smegmatis (actinobacteria).3−5 Two EGT synthetic pathways have been discovered: (1) the EgtABCDE pathway (originating from bacteria) and (2) the Egt12 pathway (originating from fungi) (Figure 2).
In EGT synthesis, S-adenosyl methionine (SAM) is initially utilized by Egt1 or EgtD in each synthetic pathway, and then L-histidine is converted to hercynine. In bacteria, hercynine is combined with γ-L-glutamyl-L-cysteine, which is synthesized from L-cysteine by EgtA; EgtB then forms γ-L- glutamyl-S-(hercyn-2-yl)-L-cysteine S-oxide, which is converted by EgtC to S-(hercyn-2-yl)-L-cysteine S-oxide (HCO) by generating L-glutamate, and finally, 2-(hydroxysulfanyl)-hercynine is generated. After several spontaneous reactions, EGT is synthesized from 2-(hydroxysulfanyl)hercynine by EgtE.6 Compared with bacterial EGT synthesis, HCO is directly synthesized from hercynine by Egt1 in fungal EGT synthesis. This synthesized HCO is converted into 2-(hydroxysulfanyl)hercynine, and EGT is synthesized via spontaneous reactions by Egt2[4].
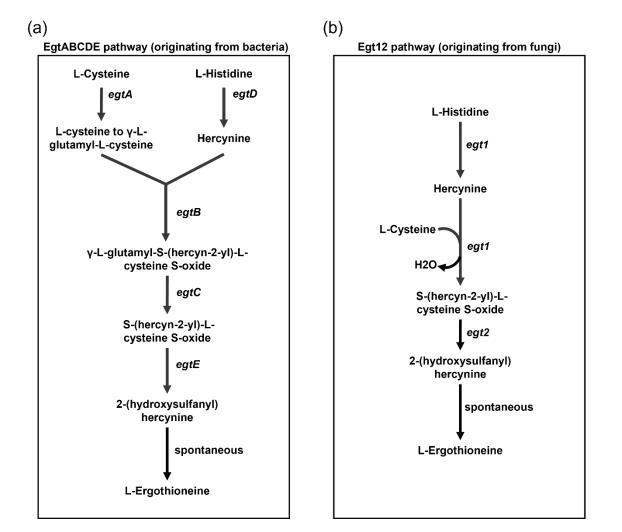
Figure 2 L-Ergothioneine synthetic pathways. (a) EgtABCDE pathway (b) Egt12 pathway
L-Ergothioneine (EGT) is a strong antioxidant used in industry, and it is commonly extracted from mushrooms; however, its production is limited. As an alternative, we developed metabolically engineered Corynebacterium glutamicum with reinforced sulfur assimilation and pentose phosphate pathways, which led to the accumulation of 45.0 and 63.2 mg/L EGT, respectively. Additionally, the overexpression of cysEKR resulted in further promoted EGT production in ET4 (66.5 mg/L) and ET7 (85.0 mg/L). Based on this result, we developed the strain ET11, in which all sulfur assimilatory, PP, and L-cysteine synthetic pathways were reinforced, and it synthesized 264.4 mg/L EGT. This study presents the first strategy for EGT synthesis that does not require precursor addition in C. glutamicum, and the production time was shortened. In addition, the synthesized EGT showed high radical scavenging activity (70.7%), thus confirming its antioxidant function. Consequently, this study showed the possibility of EGT commercialization by overcoming the limitations of industrial processes.
Toxicity and safety
Ergothioneine is a naturally occurring antioxidant and amino acid that has been shown to have a range of potential health benefits. While there is no established toxicity limit for ergothioneine, several safety evaluations have been conducted to assess its safety for human consumption. The available data suggests that ergothioneine is generally safe for human consumption, with no significant adverse effects reported in animal studies or in human clinical trials. In fact, ergothioneine is classified as a Generally Recognized as Safe (GRAS) substance by the United States Food and Drug Administration (FDA). In terms of toxicity, there have been no reports of ergothioneine toxicity in humans, and animal studies have also shown no evidence of toxicity. In fact, studies have shown that ergothioneine has a very low acute toxicity and is well tolerated by animals even at very high doses. Overall, the available evidence suggests that ergothioneine is a safe and well-tolerated dietary supplement, and may have potential health benefits as an antioxidant and anti-inflammatory agent. However, as with any dietary supplement, it is recommended to consult with a healthcare professional before taking ergothioneine or any other supplement, especially if you have any underlying health conditions or are taking medications.
Reference
1.Yadan J. C., "Matching chemical properties to molecular biological activities opens a new perspective on l-ergothioneine," FEBS Lett, Vol.596, No.10(2022), pp.1299-1312.
2.Gao Y., Zhou B. & Zhang H. et al., "l-Ergothioneine Exhibits Protective Effects against Dextran Sulfate Sodium-Induced Colitis in Mice," ACS Omega, Vol.7, No.25(2022), pp.21554-21565.
3.Fu T. & Shen L., "Ergothioneine as a Natural Antioxidant Against Oxidative Stress-Related Diseases," Frontiers in pharmacology, Vol.13(2022), p.850813.
4.Kim M., Jeong D. W. & Oh J. W. et al., "Efficient Synthesis of Food-Derived Antioxidant l-Ergothioneine by Engineered Corynebacterium glutamicum," J Agric Food Chem, Vol.70, No.5(2022), pp.1516-1524.
You may like
Related articles And Qustion
See also
Lastest Price from L-(+)-Ergothioneine manufacturers
US $0.00-0.00/KG2025-11-27
- CAS:
- 497-30-3
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $0.00-0.00/KG2025-09-29
- CAS:
- 497-30-3
- Min. Order:
- 1KG
- Purity:
- ≥99.99%(HPLC)
- Supply Ability:
- 10000KGS