Ronidazole:Application,Synthesis,Toxicity
General description
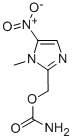
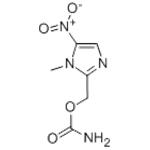
Ronidazole is a carbamate ester that is 5-nitroimidazole in which the hydrogens at positions 1 and 2 are replaced by methyl and (carbamoyloxy)methyl groups, respectively. An antiprotozoal agent, it is used in veterinary medicine for the treatment of histomoniasis and swine dysentery. It has a role as an antiprotozoal drug and an antiparasitic agent. It is a member of imidazoles, a carbamate ester and a C-nitro compound. It was first developed in the 1970s by the French pharmaceutical company Rhône-Poulenc as an antiprotozoal agent for use in veterinary medicine. It was initially developed as a more potent and effective alternative to the existing antiprotozoal drugs of the time. The drug was first introduced in Europe in the early 1980s under the brand name "Ridzol" and was initially used to treat infections caused by the protozoan parasite Tritrichomonas foetus in cattle. In the following years, it was also found to be effective against other protozoan parasites, such as Giardia and Trichomonas, in a variety of animal species, including cats, dogs, horses, and birds. Ronidazole was later approved for use in several other countries, including Australia and Canada, and is now widely used as a veterinary medication worldwide. In recent years, it has also been investigated for its potential use in human medicine, particularly in the treatment of infections caused by Helicobacter pylori, a bacterium that can cause ulcers and gastric cancer[1].
Application and Pharmacology
Ronidazole is a medication that is used in veterinary medicine to treat certain protozoal infections in animals. It is often used to treat infections caused by Giardia, a protozoal parasite that can cause diarrhea in dogs and cats. Ronidazole is also used to treat infections caused by Trichomonas, a protozoal parasite that can cause reproductive issues in birds. Pharmacologically, Ronidazole is a nitroimidazole derivative that acts by disrupting the DNA synthesis of protozoa, leading to their death. It is well-absorbed from the gastrointestinal tract and rapidly metabolized in the liver to form the active metabolite, 2-hydroxymethyl-5-nitroimidazole. This metabolite is then further metabolized to inactive metabolites that are excreted in the urine and feces. The recommended dosage of Ronidazole varies depending on the species, body weight, and the severity of the infection being treated. It is typically administered orally, either as tablets or a suspension. Treatment durations can range from several days to several weeks, depending on the specific infection being treated[2].

Figure 1 For treatment of Canker and other protozoal infections in birds (Ronidazole 6% Powder Generic)
1. Clostridioides difficile infection (CDI) is a principal cause of hospital-acquired infections and fatalities worldwide. The need for new, more potent anticlostridial agents is far from being met. Drug repurposing can be utilized as a rapid and cost-efficient method of drug development. Evaluating the activity of ronidazole, a veterinary antiprotozoal drug, as a potential treatment for CDI. Ronidazole inhibited the growth of clinical C. difficile isolates (including NAP1 and toxigenic strains) at a very low concentration (0.125 μg/mL) and showed superior killing kinetics compared with metronidazole, an anticlostridial agent from the same chemical category. In addition, ronidazole did not inhibit growth of several commensal organisms naturally present in the human intestine that play a protective role in preventing CDIs. Furthermore, ronidazole was found to be non-toxic to human gut cells and permeated a monolayer of colonic epithelial cells (Caco-2) at a slower rate than metronidazole. Finally, ronidazole outperformed metronidazole when both were tested at a dose of 1 mg/kg daily in a mouse model of CDI. Overall, ronidazole merits further investigation as a potential treatment for CDIs.
2. Efficacy of Ronidazole for treatment of cats experimentally infected with a Korean Isolate of tritrichomonas foetus. The experimental infection was confirmed by direct microscopy, culture, and single-tube nested PCR, and all cats demonstrated trophozoites of T. foetus by day 20 post-in-fection in the feces. From day 30 after the experimentally induced infection, 3 cats were treated with ronidazole (50 mg/kg twice a day for 14 days) and 3 other cats received placebo. Feces from each cat were tested for the presence of T. foetus by direct smear and culture of rectal swab samples using modified Diamond’s medium once a week for 4 weeks. To confirm the culture results, the presence of T. foetus rRNA gene was determined by single-tube nested PCR assay. All 3 cats in the treatment group receiving ronidazole showed negative results for T. foetus infection during 2 weeks of treatment and 4 weeks follow-up by all detection methods used in this study. In contrast, rectal swab samples from cats in the control group were positive for T. foetus continuously throughout the study. The present study indicates that ronidazole is also effective to treat cats infected experimentally with a Korean isolate of T. foetus at a dose of 50 mg/kg twice a day for 14 days.
3. In a group of pedigree cats (n = 17) in poor health condition housed in an animal shelter in Vienna, Austria, with a history of persistent diarrhea, Tritrichomonas foetus infection was detected by PCR. Despite pre-existing clinical conditions all cats were treated with ronidazole (30 mg/kg PO q24h for 14 days) under close observation. After treatment, 11 of 14 initially positive animals remained negative for T. foetus during the observation period (six to eight weeks post treatment) and no diarrhea was observed. During treatment, nine cats showed mild to moderate neurological disorders (incoordination, mild tremor) at least once; six of these had already shown similar signs before treatment. Ronidazole treatment of multimorbid animals is acceptable if the benefit (here: clinical resolution and release from quarantine for adoption) is high. It is hypothesized that a high degree of inbreeding is a significant risk factor for the development of tritrichomonosis in cats[3].
Synthesis
Ronidazole is an antiprotozoal drug used in veterinary medicine to treat infections caused by protozoa. Its chemical name is 1-(2-hydroxyethyl)-2-methyl-5-nitroimidazole. Here are two possible synthetic pathways for the synthesis of Ronidazole:
1.Synthesis of Ronidazole using 2-methyl-5-nitroimidazole as a starting material:
Step 1: 2-methyl-5-nitroimidazole is treated with ethylene oxide in the presence of sodium hydroxide to form 1-(2-hydroxyethyl)-2-methyl-5-nitroimidazole.
Step 2: The resulting product is purified by recrystallization to obtain pure Ronidazole.
2.Synthesis of Ronidazole using acetaldehyde as a starting material:
Step 1: Acetaldehyde is converted to acetaldehyde oxime using hydroxylamine hydrochloride in the presence of sodium acetate.
Step 2: Acetaldehyde oxime is reacted with ethyl chloroformate in the presence of pyridine to form N-ethoxycarbonylacetaldexime.
Step 3: N-ethoxycarbonylacetaldexime is then treated with sodium methoxide in methanol to form 1-(2-hydroxyethyl)-2-methyl-5-nitroimidazole.
Step 4: The resulting product is purified by recrystallization to obtain pure Ronidazole.
Both pathways involve the conversion of the starting materials to 1-(2-hydroxyethyl)-2-methyl-5-nitroimidazole, which is then purified to obtain pure Ronidazole.
Toxicity and safety
Ronidazole works by interfering with the DNA of the protozoal parasites, ultimately causing their death. Like all medications, Ronidazole can have side effects in animals, including loss of appetite, vomiting, and diarrhea. It is important to follow your veterinarian's instructions for administering Ronidazole and to watch your pet for any adverse reactions. A group of finches were accidentally overdosed with ronidazole, a 5-nitroimidazole used for treatment of trichomoniasis. Finches developed neurologic signs on the third day of treatment and were euthanized (or died). Three finches were submitted for necropsy. Focal necrosis of the cerebellar nucleus was seen in all 3 birds, as characterized by neuronal necrosis, vacuolation of the neuropil, gemistocytic astrocytosis, hemorrhage, and axonal swelling (spheroids) with demyelination. The liver from 1 finch was analyzed for ronidazole and its metabolite, 2-hydroxymethyl-1-methyl-5-nitroimidazole, by high-performance liquid chromatography–mass spectrometry. Ronidazole was detected in the liver tissue at 2,700 ng/g (wet weight), and 2-hydroxy-methyl-1-methyl-5-nitroimidazole was detected at 140 ng/g (wet weight).
Reference
1.Lim S., Park S. I. & Ahn K. S. et al., "Efficacy of ronidazole for treatment of cats experimentally infected with a Korean isolate of Tritrichomonas foetus," Korean J Parasitol, Vol.50, No.2(2012), pp.161-164.
2.AbdelKhalek A. & Seleem M. N., "Repurposing the Veterinary Antiprotozoal Drug Ronidazole for the Treatment of Clostridioides difficile Infection," Int J Antimicrob Agents, Vol.56, No.6(2020), p.106188.
3.Woods L. W., Higgins R. J. & Joseph V. J. et al., "Ronidazole toxicosis in 3 society finches (Lonchura striata)," Vet Pathol, Vol.47, No.2(2010), pp.231-23
You may like
Related articles And Qustion
See also
Lastest Price from RONIDAZOLE manufacturers
US $0.00/kg2025-11-27
- CAS:
- 7681-76-7
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- Customise

US $1.00-4.00/KG2025-09-02
- CAS:
- 7681-76-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG