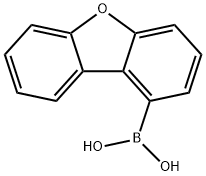
The application of 1-Dibenzofuranylboronic Acid in electronic devices
1-Dibenzofuranylboronic Acid is the reagent for electronic devices materials preparation. There are some synthesis routes to describe how do 1-dibenzofuranylboronic Acid works as the reagents for organic light emitting diode (OLED) applications.
Amir Hossain Parham and his group studied the materials for organic electroluminescent devices with electron-poor, the compounds were synthesized with some steps, such as bromination, Suzuki coupling, Ullmann coupling, Hartwig-Buchwald coupling, etc.. A suitable synthetic method is shown generally in Scheme 1 below [1].
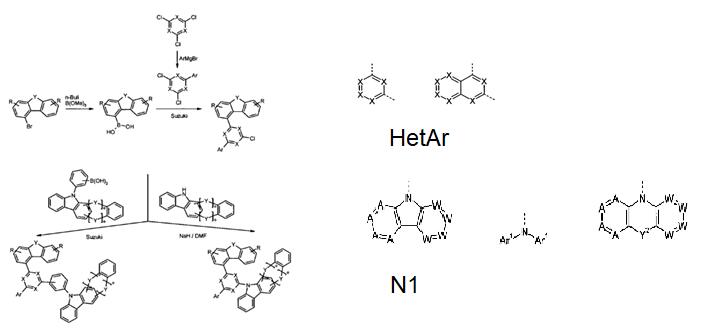
In the synthesis process, dibenzofuran or dibenzothiophen, which can be converted to the corresponding boronic acid. In the next step, the group HetAr, which carries another leaving group, can be introduced by Suzuki coupling. The HetAr is a group of the formula as shown in Scheme 1. In the last step, this further leaving group can be reacted on the group HetAr, either in a Suzuki coupling to introduce a group -LN 1, where L is an aromatic or heteroaromatic ring system, or in a CN coupling reaction, for example a Hartwig book - forest coupling, or a nucleophilic aromatic substitution reaction to introduce the group N 1.
The above synthesis route is general method, and Amir Hossain Parham team developed alternative synthetic routes in their research. The synthesis of the compounds started from 1-halo-dibenzofuran or 1-halo-dibenzothiophene, such as where the halogen is preferably bromine, characterized by the following steps:
(1) Optionally conversion of the halogen group to a boronic acid or a boronic acid derivative, such as 1-dibenzofuranylboronic Acid referred in the article. (2) Introduction of the group HetAr by a coupling reaction, in particular a Suzuki coupling. (3) Introduction of the group N 1 by a coupling reaction, in particular a Hartwig-Buchwald coupling or a nucleophilic aromatic substitution, or the group -LN 1 by a coupling reaction, in particular a Suzuki coupling.
The dibenzo [g, p] chrysene compound having a high glass transition temperature and exhibiting an excellent charge transport ability over a long period of time. 1-Dibenzofuranylboronic acid works as intermediate to synthesis the dibenzo [g, p] chrysene compound. The following scheme shows the synthesis pathway.
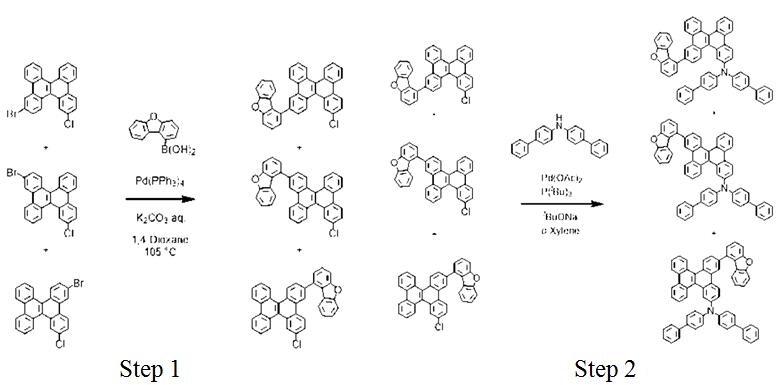
Scheme 2 The synthesis steps of dibenzo chrysene
Under a nitrogen stream, Schlenk was added to 3-bromo-15-chlorodibenzo [g, p] chrysene, 3-bromo-10-chlorodibenzo [g, p] chrysene obtained, and 3- Mixture of bromo-7-chlorodibenzo [g, p] chrysene, 1-dibenzofuranylboronic acid, tetrakis (triphenylphosphine) palladium (0), 1,4-dioxane, and aqueous potassium carbonate solution were added and stirred at 105℃ for 15 hours. After cooling to room temperature, methanol was added and stirred. The precipitated solid was collected by filtration and purified by silica gel column chromatography (chloroform / hexane) and get a mixture.
In the step 2, under a nitrogen stream, a mixture of step 1, N, N-bisbiphenylamine, sodium of tert-butoxide and o-xylene were added in Schlenk, and palladium acetate and tri-tert-butylphosphine were added to the resulting slurry reaction solution and kept stirring at 140 ℃ for 15 hours. After cooling to room temperature, pure water was added and stirred. The aqueous layer and the organic layer were separated, and the obtained organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (toluene/hexane) to obtain compounds [2]. The final dibenzo chrysene compound product exhibits excellent charge transporting ability in application.
Reference
[1]Amir Hossain Parham etc., Materials for organic electroluminescent devices, WO2016023608A1
[2] 裕太 森中, Yuta Morinaka etc., Condensed ring compound, JP2018193371A.
Related articles And Qustion
See also
Lastest Price from 1-Dibenzofuranylboronic Acid manufacturers
US $0.00-0.00/kg2025-04-04
- CAS:
- 162607-19-4
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton
![162607-19-4 dibenzo[b,d]furan-1-ylboronic acid](/ProductImageEN/2021-12/Small/a3c2b932-7634-43a1-86df-5b0d0dc719b3.jpg)
US $0.00-0.00/g2024-12-17
- CAS:
- 162607-19-4
- Min. Order:
- 1g
- Purity:
- 99.9%
- Supply Ability:
- 500kg