Tetrahydrofuran-Health Hazard and Toxicity
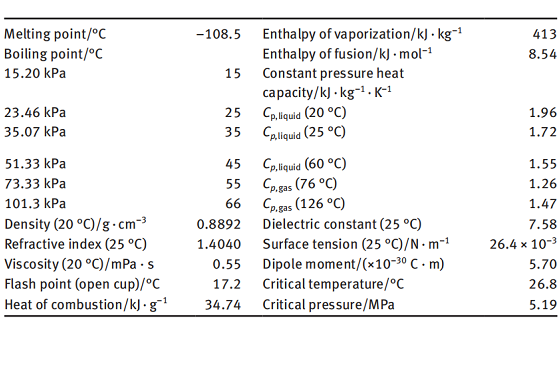
Tetrahydrofuran (THF, tetramethylene oxide, diethylene oxide, 1,4-epoxybutane, tetrahydrofurane, oxolane) is an industrial solvent widely recognized for its unique combination of useful properties. DuPont THF is better than 99.9% pure with a small (0.025-0.040 wt % ) amount of butylated hydroxytoluene (BHT, 4-methyl-2,6-di-tertbutyl phenol) added as an antioxidant. Tetrahydrofuran is a cycloaliphatic ether and is not "photochemically reactive" as defined in Section k of Los Angeles County's Rule 66 (equivalent to Rule 442 of the Southern California Air Pollution Control District). THF has an ethereal odor. The Odor Threshold is listed @ 3.8 (3M), 20-50 ppm, and 31 ppm.
Toxicity Data
LD50 oral (rat) 2880 mg/kg
LC50 inhal (rat) 21,000 ppm (3 h)
PEL (OSHA) 200 ppm (590 mg/m3)
TLV-TWA (ACGIH) 200 ppm (590 mg/m3)
STEL (ACGIH) 250 ppm (737 mg/m3)
Major Hazards
Highly flammable; forms sensitive peroxides on exposure to air, which may explode on concentration by distillation or drying.
Potential Health Effects
Inhalation:
Causes irritation to the respiratory tract. Symptoms may include coughing, shortness of breath. THF is an anesthetic agent in high concentrations. Overexposure may cause dizziness, headache, nausea and possible fluid in the lungs. May cause liver, kidney or lung injury.
Ingestion:
Causes irritation to the gastrointestinal tract. Symptoms may include nausea, vomiting and diarrhea. May cause sore throat and abdominal pain. May cause liver or kidney injury.
Skin Contact:
Causes irritation to skin. Symptoms include redness, itching, and pain.
Eye Contact:
Causes irritation, redness, and pain. Contact may cause permanent eye damage.
Chronic Exposure:
Repeated or high exposures may cause kidney or liver damage; may affect the lungs. Repeated skin exposure can cause dryness, cracking of skin and rash.
Aggravation of Pre-existing Conditions:
Persons with pre-existing skin disorders or eye problems or impaired liver or kidney function may be more susceptible to the effects of the substance.
Toxicity
The acute toxicity of THF by inhalation and ingestion is low. Liquid THF is a severe eye irritant and a mild skin irritant, but is not a skin sensitizer. At vapor levels of 100 to 200 ppm, THF irritates the eyes and upper respiratory tract. At high concentrations (25,000 ppm), THF vapor can produce anesthetic effects. Since the odor threshold for THF is well below the permissible exposure limit, this substance is regarded as having good warning properties.
Limited animal testing indicates that THF is not carcinogenic and shows developmental effects only at exposure levels producing other toxic effects in adult animals. Bacterial and mammalian cell culture studies demonstrate no mutagenic activity with THF.
Flammability and Explosibility
THF is extremely flammable (NFPA rating = 3), and its vapor can travel a considerable distance to an ignition source and "flash back." A 5% solution of THF in water is flammable. THF vapor forms explosive mixtures with air at concentrations of 2 to 12% (by volume). Carbon dioxide or dry chemical extinguishers should be used for THF fires. THF can form shock- and heat-sensitive peroxides, which may explode on concentration by distillation or evaporation. Always test samples of THF for the presence of peroxides before distilling or allowing to evaporate. THF should never be distilled to dryness.
Reactivity and Incompatibility
HF can form potentially explosive peroxides upon long exposure to air. THF may react violently with strong oxidizers and reacts vigorously with bromine and titanium tetrachloride. Polymerization can occur in the presence of cationic initiators such as certain Lewis acids and strong protic acids.
Storage and Handling
THF should be handled in the laboratory using the "basic prudent practices", supplemented by the additional precautions for dealing with extremely flammable substances. In particular, THF should be used only in areas free of ignition sources, and quantities greater than 1 liter should be stored in tightly sealed metal containers in areas separate from oxidizers. Containers of THF should be dated when opened and tested periodically for the presence of peroxides.
Accidents
In the event of skin contact, immediately wash with soap and water and remove contaminated clothing. In case of eye contact, promptly wash with copious amounts of water for 15 min (lifting upper and lower lids occasionally) and obtain medical attention. If THF is ingested, obtain medical attention immediately. If large amounts of this compound are inhaled, move the person to fresh air and seek medical attention at once. In the event of a spill, remove all ignition sources, soak up the THF with a spill pillow or absorbent material, place in an appropriate container, and dispose of properly. Respiratory protection may be necessary in the event of a large spill or release in a confined area.
Disposal
Excess THF and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines.
You may like
Related articles And Qustion
See also
Lastest Price from Tetrahydrofuran manufacturers

US $0.00/kg2025-06-18
- CAS:
- 109-99-9
- Min. Order:
- 1000kg
- Purity:
- i
- Supply Ability:
- 80MT

US $10.00/KG2025-04-21
- CAS:
- 109-99-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt