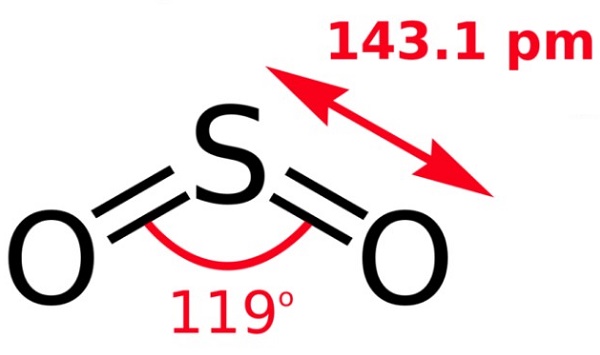
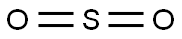Sulfur dioxide--Health Hazard and Toxicity
Sulfur dioxide is a noncombustible colorless gas at ambient temperatures with a characteristic, strong, suffocating odor. The Odor Threshold is 1.1ppm. Shipped as a liquefied compressed gas.Sulfur dioxide is used as a bleaching andfumigating agent; as a disinfectant, for treat ing wood pulp for manufacturing paper, inmetal refining, for preserving food and vegetables, and as a reducing agent. It is a majorair pollutant and is produced when soft coal,oils, or other sulfur-containing substances areburned. Automobile exhaust gases also contribute to air pollution. Sulfur dioxide in theatmosphere reacts with moisture to form sulfurous acid, or is oxidized to sulfur trioxide,which forms sulfuric acid, causing acid rain.
Toxicity Data
LC50 inhal (rat) 2520 ppm (6590 mg/m3; 1 h)
LCLO inhal (human) 1000 ppm (2600 mg/m3; 10 min)
PEL (OSHA) 5 ppm (13 mg/m3)
TLV-TWA (ACGIH) 2 ppm (5.2 mg/m3)
STEL (ACGIH) 5 ppm (13 mg/m3)
Major Hazards
Intensely irritating to the skin, eyes, and respiratory tract; moderate acute toxicity. The acute toxicity of sulfur dioxide is moderate. Inhalation of high concentrations may cause death as a result of respiratory paralysis and pulmonary edema. Exposure to 400 to 500 ppm is immediately dangerous, and 1000 ppm for 10 min is reported to have caused death in humans. Sulfur dioxide gas is a severe corrosive irritant of the eyes, mucous membranes, and skin. Its irritant properties are due to the rapidity with which it forms sulfurous acid on contact with moist membranes. When sulfur dioxide is inhaled, most of it is absorbed in the upper respiratory passages, where most of its effects then occur. Exposure to concentrations of 10 to 50 ppm for 5 to 15 min causes irritation of the eyes, nose, and throat, choking, and coughing. Some individuals are extremely sensitive to the effects of sulfur dioxide, while experienced workers may become adapted to its irritating properties. Sulfur dioxide is regarded as a substance with good warning properties except in the case of individuals with reactive respiratory tracts and asthmatics. Exposure of the eyes to liquid sulfur dioxide from pressurized containers can cause severe burns, resulting in the loss of vision. Liquid SO2 on the skin produces skin burns from the freezing effect of rapid evaporation. Sulfur dioxide has not been shown to be carcinogenic or to have reproductive or developmental effects in humans. Chronic exposure to low levels of sulfur dioxide has been shown to exacerbate pulmonary disease. Flammability and Explosibility Sulfur dioxide is a noncombustible substance (NFPA rating = 0). Reactivity and Incompatibility Contact with some powdered metals and with alkali metals such as sodium or potassium may cause fires and explosions. Liquid sulfur dioxide will attack some forms of plastics, rubber, and coatings.
Storage and Handling
Sulfur dioxide should be handled in the laboratory using the "basic prudent practices", supplemented by the procedures for work with compressed gases.
Accidents
In the event of skin contact, immediately wash with water and remove contaminated clothing. In case of eye contact, promptly wash with copious amounts of water for 15 min (lifting upper and lower lids occasionally) and obtain medical attention. If large amounts of this compound are inhaled, move the person to fresh air and seek medical attention at once.
Leaks of sulfur dioxide may be detected by passing a rag dampened with aqueous NH3 over the suspected valve or fitting. White fumes indicate escaping SO2 gas. To respond to a release, use appropriate protective equipment and clothing. Positive pressure air-supplied respiratory protection is required. Close cylinder valve and ventilate area. Remove cylinder to a fume hood or remote area if it cannot be shut off. If in liquid form, allow to vaporize.
People sensitive to sulfur dioxide
- People with lung diseases, such as asthma, chronic bronchitis, and emphysema will generally have more serious health effects at higher SO2 levels.
- Children are at higher risk from SO2 exposure because their lungs are still developing. They are also more likely to have asthma, which can get worse with SO2 exposure.
- Older adults may be more affected by SO2 exposure, possibly because they are more likely to have pre-existing lung or cardiovascular disease.
- Active people of all ages who exercise or work outdoors have higher exposure to sulfur dioxide than people who are less active.
Hawai'i Volcanoes NP visitors, residents, and park staff downwind of the volcanic SO2 emissions can be exposed to unhealthy levels of pollution. Since it is not possible to control volcanic activity, the National Park Service created a sulfur dioxide advisory program, which gives out warnings to let people know when unhealthy levels of this pollutant are present. Advisories encourage people to limit their exposure when necessary.
Disposal
Excess sulfur dioxide should be returned to the manufacturer if possible, according to your institution's waste disposal guidelines.