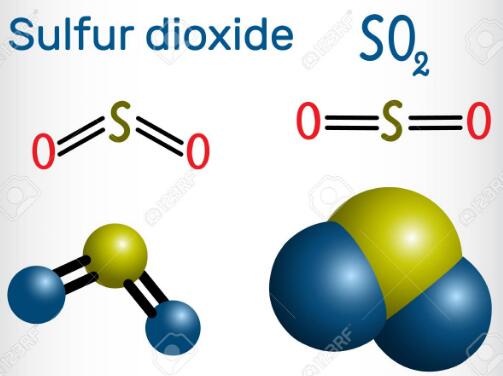
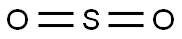The Molecular structure and polarity of Sulfur dioxide
Description
Sulfur dioxide (SO2) is a gaseous air pollutant composed of Sulfur and Oxygen. SO2 forms when sulfur-containing fuel such as coal, petroleum oil, or diesel is burned. Sulfur dioxide gas can also change chemically into sulfate particles in the atmosphere, a significant part of fine particle pollution, which can blow hundreds of miles away. As a toxic gas and air pollutant, the toxic effects of SO₂ have been extensively studied. Oxidative damage due to SO₂ can occur in multiple organs. Inhaled SO₂ can also cause chromosomal aberrations, DNA damage, and gene mutations in mammals. However, recent studies have shown that SO₂ has a vasorelaxant effect and ameliorates pulmonary hypertension.
Molecular structure
Sulfur dioxide consists of one atom of Sulfur (S) and two atoms of Oxygen (O). In the SO2 molecule, the Sulfur has 6 electrons in its vacant shell, and Oxygen also has 6 electrons in its vacant shell. Moreover, after bonding in the SO2 molecule, the unequal charge remains on Sulfur and Oxygen. 2 unbonded electrons remain on Sulfur, and 4 electrons on both Oxygen atoms.
The electron density is not equally shared on both sides. Oxygen is more electronegative than Sulfur, so it more strongly attracts the shared electron cloud from each S=O bond. Therefore, each S=O bond is polar. The central Sulfur has a partial positive charge, while each Oxygen carries a partial negative charge.
According to the difference in electronegativity between sulfur and oxygen atoms. Each S=O bond is polar and has a dipole moment equal to 0.82 Debye. The dipole moment is a vector quantity. Its direction is from a polar bond's positive to a negative center. Thus, in S=O, it directs from the Sδ+ atom to the Oδ- atom.
According to the Valence Shell Electron Pair Repulsion (VSEPR) theory of chemical bonding, SO2 is an AB2E-type molecule. Each S=O is considered one bond pair. So, there are two bond (B) pairs and 1 lone (E) pair around the central Sulfur (A) atom. The lone pair situated on Sulfur repels the bonded pair of electrons. Lone pair-bond pair repulsions are greater than bond pair-bond pair repulsions. So, the lone pair pushes the two bond pairs closer to each other, and the bond angle in an SO2 molecule decreases. This contributes to the bent or V-shape of the SO2 molecule. 119° bond angle is present in the polar SO2 molecule as opposed to 120° in trigonal planar molecules with three bond pairs and no lone pair.
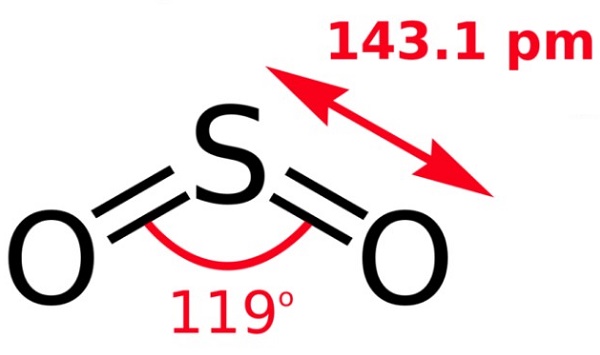
The asymmetrical, bent, or V-shaped geometrical structure of SO2 maintains the molecule's polarity intact. Thus, SO2 is a polar molecule with a net dipole moment more significant than zero. A lone electron pair on Sulfur repels Oxygen’s lone pairs, making SO2 a bent structure with a bond angle 119°.