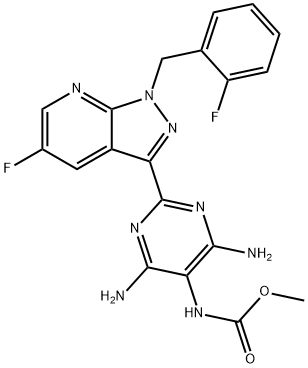
Synthesis of Vericiguat
Synthesis of Vericiguat
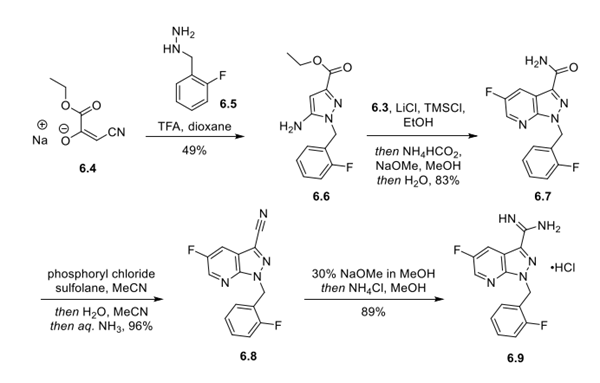
The Vericiguat synthesis was initiated with the key fluoroacrylaldehyde intermediate required to construct the fluoropyrazolopyridine core of vericiguat. The specific synthesis steps are as follows:
Step 1: Preparation of α-Fluoro-β-(dialkylamino)acrylaldehyde Intermediate
An SN2 reaction between 2,2,3,3-tetrafluoropropyltosylate (6.1) and morpholine provided intermediate 6.2 in 85% yield. The amine of 6.2 was then converted to a water-soluble quaternary ammonium salt by methylation with methyl methanesulfonate. Treatment of the quaternary amine with aqueous sodium hydroxide resulted in elimination of HF. Finally, addition of morpholine and triethylamine to the reaction solution resulted in a series of addition−elimination processes (first addition of morpholine and water and then elimination of NMM and 2 equiv of HF) that delivered the α-fluoro-β- (dialkylamino)acrylaldehyde (6.3) in 83% yield over 3 telescoped steps.
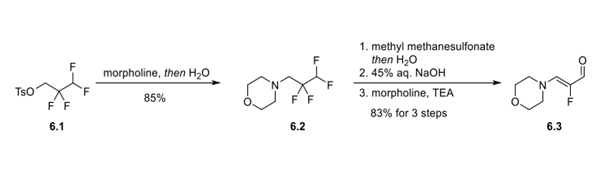
Step 2: Preparation and Functionalization of the Fluoropyrazolopyridine Core
Ethyl β-cyanopyruvate sodium salt (6.4) underwent acid-mediated condensation with hydrazine 6.5 in dioxane to deliver aminopyrazole 6.6 in 49% yield. Next, 6.6 was condensed with intermediate 6.3 in the presence of LiCl and TMSCl in EtOH to form the fluoropyrazolopyridine ring system. In the same pot, the ethyl ester was converted to the primary amide by the addition of ammonium formate and sodium methoxide in MeOH, providing 6.7 in 83% yield over 2 steps. Dehydration of the primary amide with phosphoryl chloride in sulfolane delivered nitrile 6.8 in 96% yield. The nitrile was then converted into the guanidine hydrochloride (6.9), via the imidate, in 89% yield by treatment with sodium methoxide followed by ammonium chloride in MeOH.
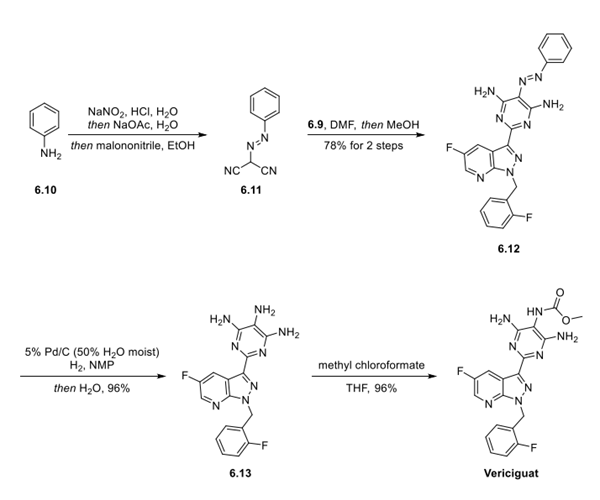
Step 3: Preparation of Vericiguat
Completing the synthesis of vericiguat (6) started with the preparation of intermediate 6.11 from the diazonium salt of aniline (6.10) and malononitrile. The bisnitrile 6.11 was then condensed with intermediate 6.9 in DMF to deliver 6.12 in 78%over 2 steps. Reduction to the amine (6.13) was achieved by catalytic hydrogenation in 96% yield. Finally, methyl chloroformate reacted selectively with the C5 amino group to deliver vericiguat (6) in a 96% yield.
You may like
Related articles And Qustion
See also
Lastest Price from vericiguat manufacturers

US $2.00-5.00/kg2025-06-18
- CAS:
- 1350653-20-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100kg

US $0.00/kg2025-06-02
- CAS:
- 1350653-20-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg