How is Serdexmethylphenidate synthesised?
Synthesis of Serdexmethylphenidate
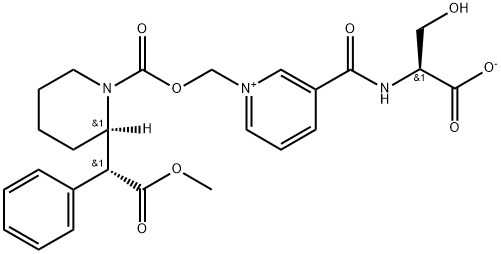
Serdexmethylphenidate is synthesised derived from dexmethylphenidate hydrochloride and prepared by a series of reactions including coupling and acidification. The specific synthesis steps are as follows:
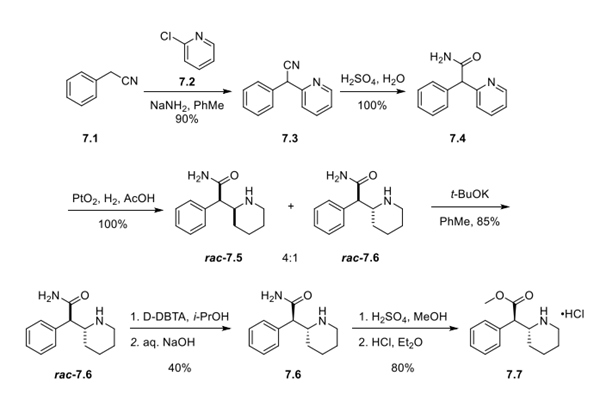
Step 1: Preparation of Dexmethylphenidate Hydrochloride
Coupling of benzyl cyanide 7.1 with 2-chloropyridine 7.2 gave racemic nitrile 7.3, which was hydrated to the corresponding amide 7.4 by using concentrated sulfuric acid. Reduction of the pyridine was then achieved under hydrogenation conditions with PtO2 as a catalyst, giving rise to a 4:1 mixture of erythro:threo diastereomers (rac-7.5:rac-7.6). Treatment of this mixture with potassium t-butoxide in toluene enabled the epimerization of the undesired erythro isomer rac-7.5 into the desired threo isomer rac-7.6. A chiral salt resolution was then performed using dibenzoyl-D-tartaric acid (D-DBTA), and the free base 7.6 was generated following a salt break with aqueous NaOH. Although enantiomeric excess (ee) values are not provided in this particular patent, ee values of >99% have been described in subsequent publications for this step. Finally, conversion of the amide to the ester was accomplished with concentrated sulfuric acid in MeOH, making way for treatment with ethereal HCl to yield hydrochloride salt 7.7.
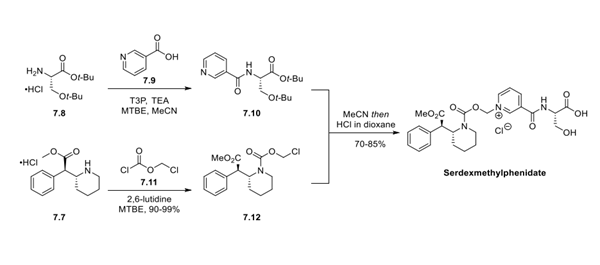
Step 2: Preparation of Serdexmethylphenidate
Initial preparation of the bis-O-t-Bu-protected nicotinoyl-L-serine motif 7.10 was accomplished in an undisclosed yield by the coupling of commercially available O-t-Bu-protected L-serine 7.8 and nicotinic acid 7.9 utilizing propylphosphonic anhydride as a coupling reagent. The carboxymethylene unit was installed by treating dexmethylphenidate hydrochloride 7.7 with chloroformate 7.11 in the presence of base. The reaction mixture was concentrated before treating the intermediate 7.12 with pyridine 7.10 in MeCN. Subsequent acidification with HCl in dioxane and crystallization from MIBK/heptane afforded serdexmethylphenidate.