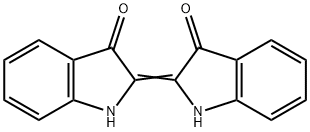
Synthesis of Indigo Dye
Indigo dye has been produced commercially for hundreds of years, but its history extends to pre-Roman Britain. This article will introduce a synthesis method of indigo dye.
2-Nitrobenzaldehyde dissolves in propanone to form a pale yellow solution. When sodium hydroxide solution is added, the solution darkens after a few seconds and a purple precipitate of indigo forms.
The synthesis is very simple and quick to perform, yet it is mechanistically complex, involving a series of condensations, disproportionations and oxidations. The sequence of reactions is given in the figure below, together with the structures of the two forms of indigo.
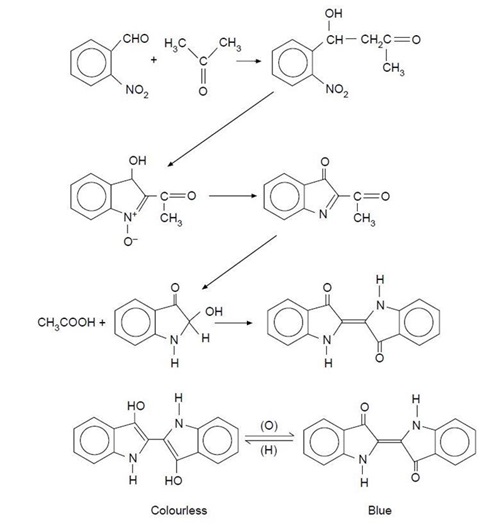
There is one unusual step which produces ethanoic acid and what appears to be a hydroxy-derivative of indoxyl prior to the final oxidation to form indigo (indigotin). It is worthwhile pointing out that it took Baeyer 14 years (from 1865 to 1879) to find a route for the original synthesis of indigo and even when he did, it took him another four years to deduce the correct formula (in 1883).
The indigo can be filtered off and then dissolved in an alkaline solution of sodium dithionite to form the colourless, soluble form (leucoindigo). A piece of cotton cloth dipped into this solution and then exposed to air produces the indigo-dyed fabric – this is the procedure used in vat dyeing.
You may like
Related articles And Qustion
See also
Lastest Price from Indigo manufacturers

US $10.00/KG2025-04-21
- CAS:
- 482-89-3
- Min. Order:
- 100KG
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $79.00-38.00/kg2025-04-21
- CAS:
- 482-89-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton