Synthesis of 2,4-Dichlorothieno[3,2-d] pyrimidine
2,4-Dichloro-thieno[3,2-d] pyrimidine is one of the active intermediates that is a must for new antitumor medications. There are two chloride atoms in this compound that are easy for other functional groups to replace bringing various unit processes, which are often used in the kernel steps for synthesizing the new anticancer medicines mentioned above and is attracting more concern [1]. Therefore, research of this synthesis process is of important meaning. Because of the low yield, slow reaction, small productivity, high consumption of solvent, and high cost, traditional methods are not ideal for industrial scale.
Dong Liuyu and coworkers used 3-amino-2-methoxycarbonylthiophene (A) as raw material and adopted mainly process to synthesize the target product 2,4-dichloro-thieno [3,2-d]pyrimidine (C). As shown in scheme 1, the reaction process has two steps, and the additional purification step is the key for the reaction. The raw target product was refined with a mixed solution of ethyl alcohol and chloroform (lacquer solvent) and then an essential final product is obtained with the purity up to 99.5%, which is significantly higher than that of the traditional processes.

Zhihui Zhou and team synthesized 2,4-Dichloro-thieno[3,2-d] pyrimidine as prepare 2-chloro-4-(3-nitrophenoxy) thieno [3, 2-d] pyrimidine [2].
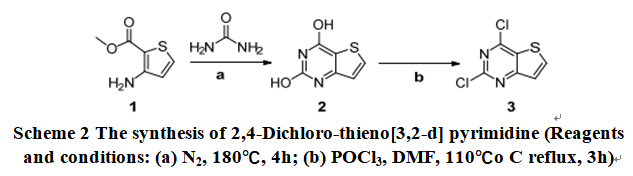
Put the mixture of 2-amino-4-fluoro benzoic acid and urea in three flasks. After stirring for 4h with mechanical agitation at 180℃, the reaction was complete by TLC analysis. Slightly cool down, a saturated aqueous NaHCO3 solution was added, and adding 10% NaOH solution. Filtration, the filter cake was transferred to a beaker, and slowly adding the dilute HCl solution and stir the 10min. filtration. The filter cake was washed with saturated aqueous NaHCO3 solution, dried to obtain a white solid [3, 2-d] pyrimidine-2, 4-diol (2 in scheme 2). The second step is the preparation for 2, 4-dichlorothieno[3,2-d] pyrimidine (3). A mixture of thieno[3,2-d] pyrimidine-2,4-diol (2), POCl3 and DMF was heated and stirred for 3h at 120℃, and the reaction was monitored by TLC. The mixture was concentrated under reduced pressure to afford the product as viscous oil. Then, the mixture was transferred to a beaker, ice water acetate was added slowly with stirring. Filtration, the filter cake was washed with ice-water, dried to obtain a brown solid 2,4-Dichloro-thieno[3,2-d] pyrimidine.
![2,4-Dichlorothieno[3,2-d] pyrimidineSynthesis of 2,4-Dichlorothieno[3,2-d] pyrimidine](https://www.chemicalbook.com/StructureFile/ChemBookStructure9/GIF/CB0386346.gif)