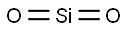Synthesis method and application of Silicon dioxide
Background and overview
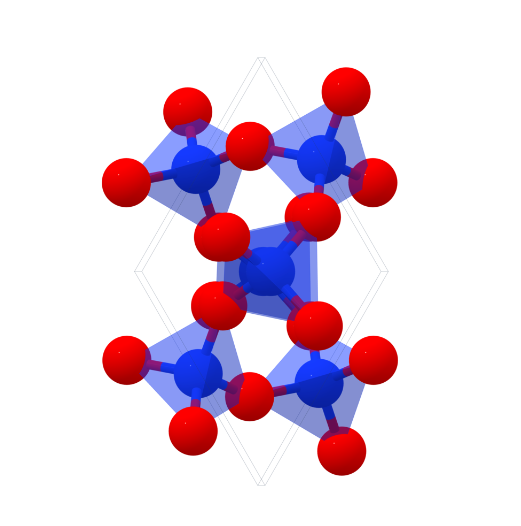
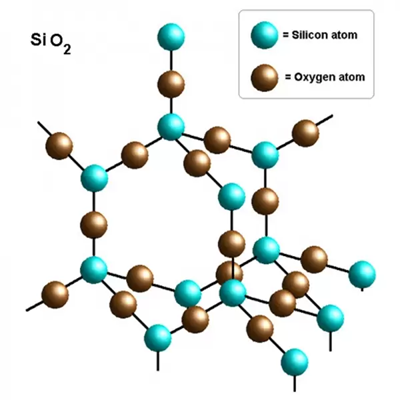
Silica is an inorganic substance with the chemical formula SiO2. Silicon atoms and oxygen atoms are arranged in long-range order to form crystalline silica, and short-range order or long-range disorder form amorphous silica. In the silicon dioxide crystal, the silicon atom is located in the center of the regular tetrahedron, and the four oxygen atoms are located at the four corners of the regular tetrahedron. Many such tetrahedra are connected by the oxygen atoms at the corners. The two tetrahedra are in common, ie each oxygen atom is bound to two silicon atoms. The simplest form of silicon dioxide is SiO2, but SiO2 does not represent a simple molecule (it only represents the ratio of the number of atoms of silicon and oxygen in the silicon dioxide crystal). Pure natural silica crystal, is a hard, brittle, insoluble, colorless and transparent solid.

Preparation【1】
Step 1. Reduction reaction process: the raw material triphenylphosphine oxide is weighed and added to the material preparation kettle, the solvent toluene is weighed and added to the material preparation kettle, and trichlorosilane is added dropwise under normal pressure. The reaction kettle was slowly heated to 80°C, and the reflux reaction was carried out under the heat preservation condition. After the heat preservation reflux reaction was completed, unreacted trichlorosilane and toluene were distilled off. After the distillation was completed, the reaction kettle was cooled to below 30°C;
Step 2. Hydrolysis reaction process: first weigh the purified water and add it to the hydrolysis kettle, open the hydrolysis kettle to stir, then open the reduction reaction kettle discharge valve, and drop the reduction reaction product into the hydrolysis reaction kettle through a closed pipeline to conduct hydrolysis. reaction, control the rate of dripping, so that the temperature in the kettle does not exceed 35 ℃, after the completion of the dripping, the reaction product precipitated in the hydrolysis reaction process is separated by a centrifuge to obtain solid-phase silica;
Step 3: Desorb the silicon dioxide, the main process is as follows: adding a quantitative sodium hydroxide aqueous solution into the alkali dissolution kettle, starting stirring, then adding the silicon dioxide filter cake produced by the centrifugal process, and stirring until the silicon dioxide is completely dissolved , silicon dioxide and sodium hydroxide solution react to generate sodium silicate and water, control the temperature not to exceed 80 ℃, add hydrochloric acid dropwise to adjust the pH value to be controlled between 5-6, keep the temperature for 3 hours and measure the pH value unchanged, filter by pressure, The filter cake is washed with hot water to obtain the silica product.
Use
Silica is a raw material for the manufacture of glass, quartz glass, water glass, optical fiber, important components of the electronic industry, optical instruments, handicrafts and refractory materials, and is an important material for scientific research.
When the crystalline silica is perfect, it is crystal; when silica is gelled and dehydrated, it is agate; when the water-containing colloid of silica is solidified, it becomes opal; when the crystallite of silica is smaller than a few microns, it is composed of chalcedony, flint, secondary Quartzite. It is a mineral resource with very stable physical and chemical properties. The crystal belongs to the oxide mineral of the trigonal system, that is, low-temperature quartz (α-quartz), which is the most widely distributed mineral species in the quartz group. Quartz in a broad sense also includes high temperature quartz (beta-quartz). Quartz block, also known as silica, is mainly the raw material for the production of quartz sand (also known as silica sand), as well as the raw material for quartz refractories and sintering ferrosilicon.
In addition, silica can also be used as a lubricant and is an excellent flow promoter, mainly used as a lubricant, anti-sticking agent, and glidant. It is especially suitable for the granulation of oil and extract medicines, and the granules made have good fluidity and compressibility. It can also be used as a glidant in direct compression. As a disintegrant, it can greatly improve the fluidity of granules, increase the bulk density, increase the hardness of the prepared tablet, shorten the disintegration time limit, and improve the dissolution rate of the drug. It can be used as an internal desiccant in the manufacture of granules to enhance the stability of the drug. It can also be used as a filter aid, a clarifying agent, a defoaming agent, a suspending agent and a thickening agent for liquid preparations.
References
【1】CN113860317A
You may like
Related articles And Qustion
Lastest Price from Silicon dioxide manufacturers

US $150.00/kg2025-11-18
- CAS:
- 7631-86-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20tons

US $150.00/kg2025-11-18
- CAS:
- 7631-86-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20tons