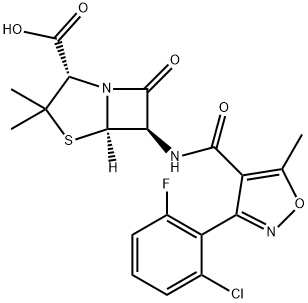
Isoxazolyl Penicillins: Antimicrobial Activity, Susceptibility, Administration and Dosage Clinical Uses etc.
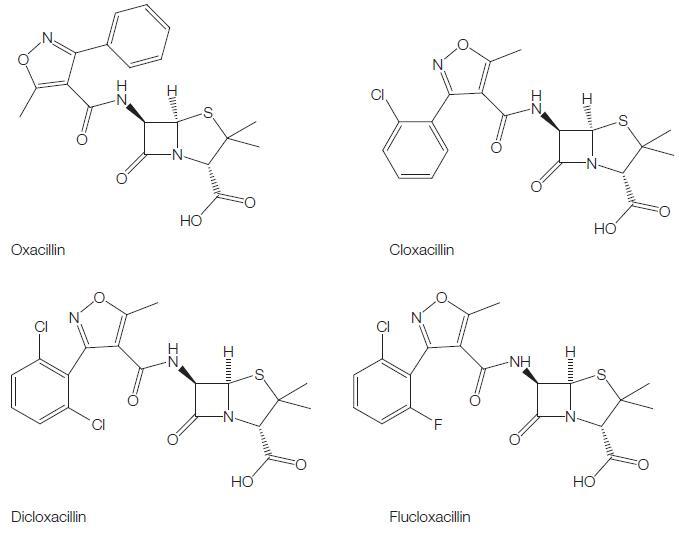
These semisynthetic compounds, derived from the penicillin nucleus, 6-APA, are all closely related, being 3:5 disubstituted 4-isoxazolyl penicillins. They combine the property of resistance to staphylococcal b-lactamase with resistance to gastric acidity. Similar to methicillin and nafcillin, they are effective antistaphylococcal agents, but they can be administered orally. Four such penicillins are available as described below (see Figure 5.1).
ANTIMICROBIAL ACTIVITY
a. Routine susceptibility
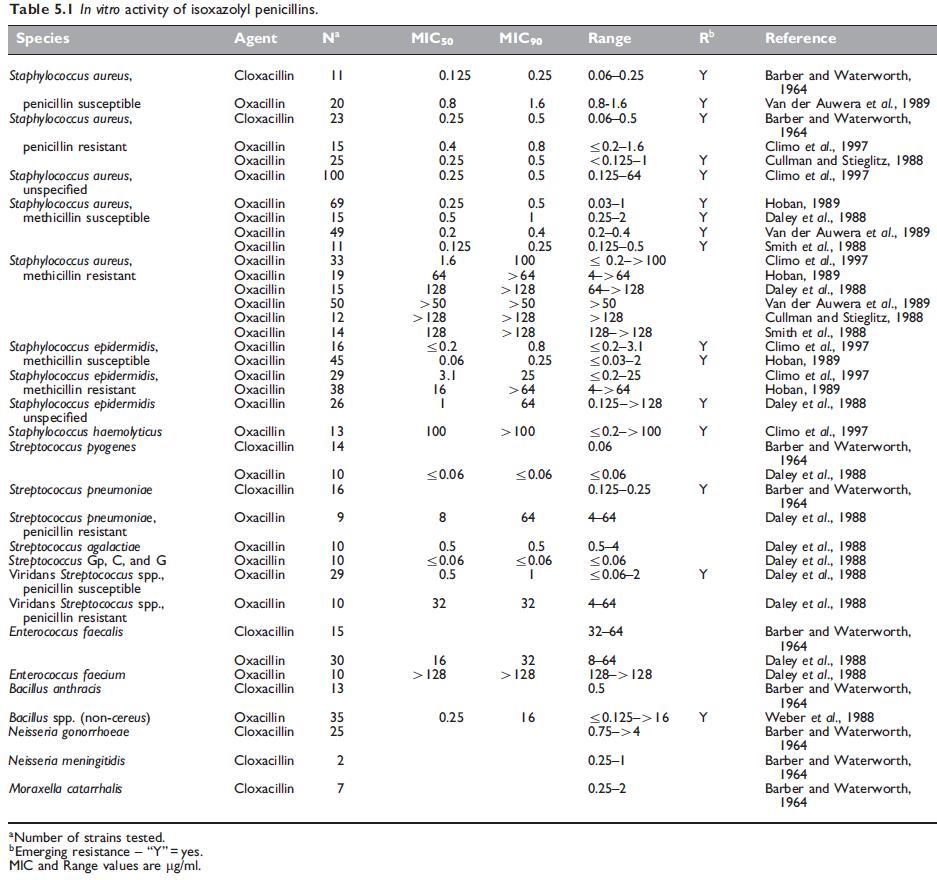
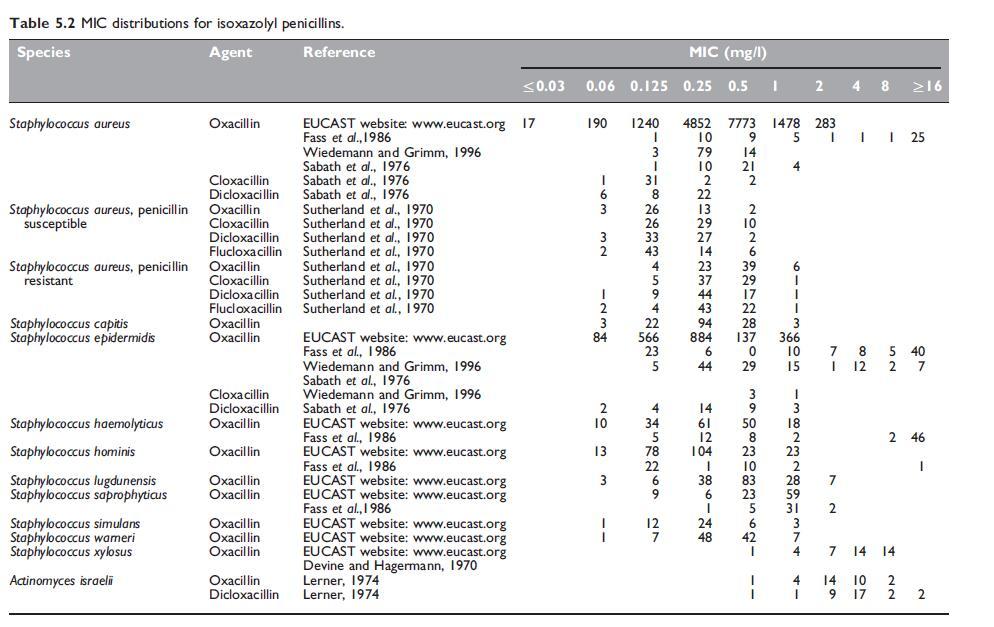
The antibacterial spectrum of the isoxazolyl penicillins is similar to that of methicillin and nafcillin. Isoxazolyl penicillins are active against Gram-positive cocci, such as wild-type Staphylococcus aureus, S. epidermidis, Streptococcus pyogenes, Streptococcus pneumoniae, viridans streptococci, and many species of Gram-positive bacteria, but Enterococcus species and Bacillus cereus are resistant. The Neisseria species are the only Gram-negative bacteria susceptible to these drugs.
b. Emerging resistance and cross-resistance
Methicillin-resistant S. aureus
Cross-resistance occurs between methicillin and all other penicillinaseresistant penicillins, so that methicillin-resistant strains of S. aureus and S. epidermidis are also resistant to isoxazolyl penicillins (Sutherland et al., 1970; Richmond et al., 1977).
Borderline oxacillin-resistant S. aureus
These S. aureus strains appear to hyperproduce b-lactamase, but other mechanisms may also be involved. Woods and Yam (1988) found that the MICs of oxacillin against these strains were 1–2 mg/l. The minimum bactericidal concentrations (MBCs) were higher, but the bactericidal testing results were markedly influenced by the technique employed. Sierra-Madero et al. (1988) found that the MICs of these strains varied from 1 to 4 mg/l and considered that oxacillin may well be less effective clinically for treatment of infections caused by these strains.
Penicillin-tolerant S. aureus
These strains have a deficiency in an autolytic enzyme on their cell surface, which appears to be necessary before any penicillins, including penicillinase-resistant penicillins, can exert a bactericidal effect. Oxacillin tolerance in S. aureus can also be due to enhanced secretion of an autolysin inhibitor, such as lipoteichoic acid (Raynor et al., 1979).
Streptococcus pneumoniae
Alterations in penicillin binding proteins (PBPs) in S. pneumoniae have a greater effect on isoxazolyl penicillins than on penicillin G, amoxicillin, or third-generation cephalosporins (Klugman, 1990).
This is the main reason for the use of oxacillin to screen for reduced penicillin susceptibility in this species (MIC W0.06 mg/l), as is recommended by internationally recognized susceptibility testing methods, such as those of the Clinical and Laboratory Standards Institute (Clinical and Laboratory Standards Institute, 2008).
MECHANISM OF DRUG ACTION
The isoxazolyl penicillins, like other penicillins, inhibit peptidoglycan synthesis by inhibiting the transpeptidase enzymes PBP1a, 1b, and 2. The principal target appears to be the bifunctional enzyme PBP2.
MODE OF DRUG ADMINISTRATION AND DOSAGE
a. Adults
Oral administration
Unlike methicillin and nafcillin, isoxazolyl penicillins are acid stable and can be administered orally. The usual oral dosage of these drugs is 500 mg 6-hourly in adults, and for children 50 mg/kg body weight per day, given in four divided doses. The dose should be administered about 1 hour before meals for optimal absorption (Sutherland et al., 1970).
b. Newborn infants and children
The usual pediatric doses are 25–50 mg/kg 6-hourly. Children weighing more that 40 kg should be given adult doses. Conventional doses for isoxazolyl penicillins in neonates have been in the range of 25–50 mg/ kg every 8–12 hours. However, taking into account the kinetics and protein binding in this age group, doses of flucloxacillin of 25 mg/kg 4-hourly have recently been recommended (Pullen et al., 2006).
PHARMACOKINETICS AND PHARMACODYNAMICS
a. Bioavailability
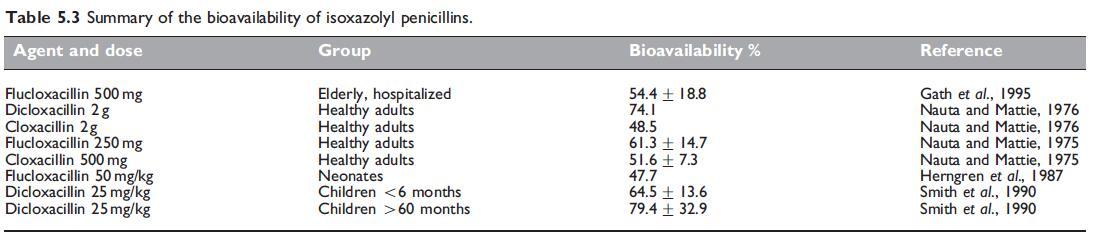
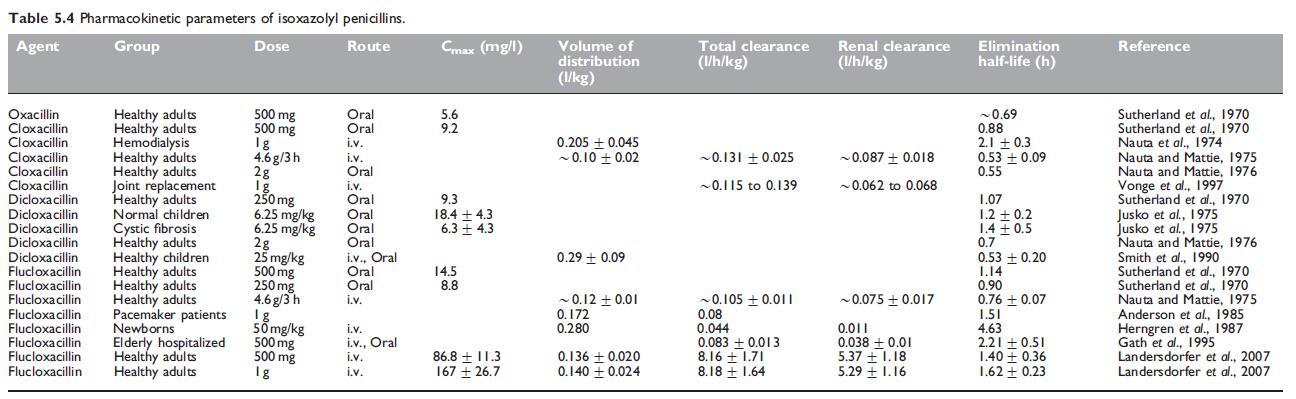
Dicloxacillin and flucloxacillin have the best oral bioavailability of all the isoxazolyl penicillins, generally above 50%. Cloxacillin has either the same or slightly lower bioavailability (Paton, 1986), whereas bioavailability of oxacillin is the lowest at around 30%. The pharmacokinetic parameters for the isoxazolyl penicillins are summarized in Table 5.4. These drugs have relatively small overall volumes of distribution, although the volume of distribution of free drug, at least for flucloxacillin, is considerably higher (Anderson et al., 1985; Herngren et al., 1987). The elimination half-lives of oxacillin and cloxacillin are shorter than 1 hour, while those of dicloxacillin and flucloxacillin are greater than 1 hour.
b. Drug distribution
Oral administration
When a 0.5-g oral dose of oxacillin is given, a peak serum level of about 4 mg/l is reached in 30–60 minutes (Hammerstrom et al., 1967). Thereafter, the serum concentration falls, but significant levels are maintained for 4–6 hours. Serum levels after cloxacillin are about twice as high as those obtained with oxacillin (see Table 5.4) (Turck et al., 1965; Sutherland et al., 1970). Oral dicloxacillin produces serum levels approximately twice as high as cloxacillin (Gravenkemper et al., 1965), as does flucloxacillin (Table 5.4).
c. Clinically important pharmacokinetic and pharmacodynamic features
Similar to other b-lactams, the clinical efficacy of isoxazolyl penicillins is thought to be related to the duration that their concentrations are above the MIC of the relevant pathogen in the site of infection.
d. Excretion
Isoxazolyl penicillins are mainly excreted in the urine. After oral administration of cloxacillin, about 30% of the dose is excreted in this way (Stewart, 1965); a higher percentage of the dose is recoverable when it is administered i.m. Compared with cloxacillin, oral oxacillin is excreted to a lesser extent via the kidney, partly because of its poorer absorption and partly because more oxacillin is cleared by other mechanisms. Larger amounts of dicloxacillin and flucloxacillin are excreted in urine after oral administration because the absorption of these drugs is better than that of cloxacillin (Sutherland et al., 1970).
e. Drug interactions
Isoxazolyl penicillins have relatively few drug interactions, and none are considered predictable. There are occasional reports of interaction between oxacillin and methotrexate, with the former reducing the clearance of methotrexate and in one case leading to significant methotrexate toxicity (Titier et al., 2002). This interaction does not occur with flucloxacillin (Herrick et al., 1996). One case of reduced phenytoin levels resulting in status epilepticus has been attributed to oral oxacillin (Fincham et al., 1976). Dicloxacillin appears to reduce the effects of warfarin and prolong prothrombin times (Krstenansky et al., 1987; Mailloux et al., 1996). It is not known if this is a class effect, although it has also been described with nafcillin.
TOXICITY
a. Hypersensitivity reactions
These drugs are generally contraindicated in penicillin-allergic patients, because they may evoke all the hypersensitivity reactions caused by penicillin G. Instances of patients who developed allergic reactions to cloxacillin but tolerated other b-lactams have been reported (Dominguez-Ortega et al., 2006).
b. Gastrointestinal side-effects
Oral administration of isoxazolyl penicillins may cause nausea and diarrhea, which only occasionally necessitates cessation of treatment. Antibiotic-associated colitis due to Clostridium difficile can be caused by these drugs; toxin-producing C. difficile was isolated from the feces of one child who developed watery diarrhea with i.v. oxacillin therapy, and from another child who developed diarrhea following 4 days of oral dicloxacillin (Brook, 1980).
c. Drug fever
This can occur with methicillin and other anti-staphylococcal penicillins; it is abrupt in onset and the patient usually appears otherwise relatively well. It rapidly resolves when the drug is stopped, and may recur later if another penicillin analog is administered (Yow et al., 1976)
d. Hepatotoxicity
Oxacillin occasionally causes fever, nausea, and vomiting associated with abnormal liver function tests, mainly elevated serum glutamic oxaloacetic transaminase (SGOT) levels (Dismukes, 1973; Onorato and Axelrod, 1978). Increases in liver enzymes are seen more commonly (Heldman et al., 1996). Liver biopsy may show a nonspecific hepatitis (Bruckstein and Attia, 1978). Some patients remain asymptomatic and anicteric, the only abnormalities being elevated serum enzymes and sometimes eosinophilia (Olans and Weiner, 1976). Reversible cholestatic hepatitis occurred in one patient (Ten Pas and Quinn, 1965).
e. Neurotoxicity
This may occur if very large doses are given i.v., especially to patients with renal failure. Conway et al. (1968) reported a patient who convulsed while receiving 18 g of i.v. cloxacillin per day, but who also had renal functional impairment and was concurrently receiving cephaloridine in a dose of 2 g daily. Malone et al. (1977) described a patient with acute bacterial endocarditis and impaired renal function who convulsed while receiving 16 g oxacillin i.v. per day. In this patient, predose oxacillin serum level was 270 mg/l, serum level 1 hour after a dose was 340 mg/l, and the CSF level was 70 mg/l.
f. Neutropenia
This has been noted particularly with oxacillin (Maraqa et al., 2002), but it can occur with the other isoxazolyl penicillins and with all b-lactam antibiotics. Leventhal and Silken (1976) described four children who developed marked neutropenia during the third week of treatment with i.v. oxacillin in a dose of 200 mg or more per kg per day.
g. Nephritis
Interstitial nephritis, similar to that seen with methicillin, has been observed with flucloxacillin (Bakker et al., 1995), cloxacillin (Garcia´- Ortiz et al., 1992), and dicloxacillin (Hansen, 1987).
h. Kernicterus
Animal studies suggested that flucloxacillin, similar to sulfonamides, may displace bilirubin from its binding sites on albumin. It is possible that this drug, if used in the jaundiced neonate, may cause kernicterus (Hanefeld and Ballowitz, 1976).
However, it is generally safe to administer to neonates.
i. Risks in pregnancy and fetal toxicity
Isoxazolyl penicillins are generally safe in pregnancy and are associated
with no known fetal toxicities or teratogenicity.
CLINICAL USES
Isoxazolyl penicillins are used primarily for the treatment of penicillinresistant methicillin-susceptible staphylococcal infections of all grades of severity; hence all the following indications relate to this pathogen. With the possible exception of orally administered oxacillin (with its lower bioavailability), all the penicillinase-resistant penicillins, including the isoxazolyl penicillins, are similarly effective for the treatment of staphylococcal infections when dosed appropriately.
a. Mild to moderate S. aureus infections
Superficial pyodermas are often treated with oral isoxazolyl penicillins (Barton et al., 1988; Amaya-Tapia et al., 1993; Rodriguez-Solares et al., 1993; Bernard et al., 1997; Chosidow et al., 2005). Cellulitis is often managed with an i.v. isoxazolyl penicillin initially. This is often combined with penicillin G to treat S. pyogenes, but it makes no difference to the outcome (Leman and Mukherjee, 2005), confirming that isoxazolyl penicillins alone can effectively cover S. aureus and S. pyogenes in this setting. Oral cloxacillin has been shown to be effective for the treatment of staphylococcal infections of moderate severity (Stewart, 1962; Turck et al., 1965). For moderately severe complicated skin and skin structure infections, oxacillin i.v. followed by dicloxacillin orally has been shown to be effective (Stevens et al., 2000).
b. Severe S. aureus infections
Results similar with those obtained with methicillin or nafcillin have been obtained with oxacillin administered in large doses parenterally (6–18 g daily for adults) for the treatment of severe staphylococcal infections, including severe pneumonia, meningitis, and endocarditis (Klein et al., 1963; Abrams et al., 1979; Rajashekaraiah et al., 1980; Watanakunakorn, 1987; Quintiliani and Cooper, 1988). Some clinical studies have suggested that the oxacillin–rifampicin combination may be slightly superior to oxacillin therapy alone for patients severely ill with S. aureus sepsis (Van der Auwera et al., 1983; Van der Auwera et al., 1985). Further studies are needed to confirm this. Parenteral cloxacillin and flucloxacillin are also satisfactory for the treatment of severe staphylococcal infections (Williams, 1982; Lacey, 1983; Eykyn, 1987), and have been successfully used as outpatient i.v. therapy (Wynn et al., 2005).
c. S. aureus endocarditis
High doses of an intravenous isoxazolyl penicillin are standard treatment for acute bacterial endocarditis caused by penicillin-resistant S. aureus. Therapy is frequently combined with gentamicin, extrapolating from a study of nafcillin plus gentamicin (Korzeniowski et al., 1982). However, it is still uncertain whether patients with staphylococcal endocarditis, due to either tolerant or nontolerant strains, would benefit from the addition of gentamicin to the treatment regimen (Karchmer, 1988). The addition of gentamicin is often associated with increased nephrotoxicity (Cosgrove et al., 2009).
d. Bone and joint infections
Isoxazolyl penicillins play a major role in the inpatient and outpatient treatment of acute bone and joint infections. They are considered standard therapy in acute osteomyelitis in children, and are the drugs of choice if S. aureus is proven. It is not clear that they are superior to other agents (Lazzarini et al., 2005). They are effective as prolonged outpatient treatment of chronic osteomyelitis (Bell, 1968; Bell, 1976; Black et al., 1987; Raber et al., 1996; Howden and Richards, 2001).
e. Surgical prophylaxis
S. aureus is the common cause of wound and postoperative infection after clean surgery. In orthopedic surgery (hip replacement and internal fixation of some fractures), flucloxacillin or cloxacillin in doses of 1–2 g given 1–2 hours preoperatively i.m. or just before the operation i.v., has been recommended. Some clinicians give repeated doses of these drugs postoperatively, but a single dose probably suffices and prophylaxis should not exceed a three-dose course (Unsworth et al., 1978; Hirschmann and Inui, 1980; Norden, 1983). Some authors consider that cloxacillin or flucloxacillin alone may not be sufficient in this situation, as Gram-negative organism infection and even anaerobic infections are being encountered more commonly after orthopedic operations.
f. Staphylococcal toxic shock
The clinical entity of the toxic shock syndrome results from a toxin or toxins elaborated by S. aureus, the infection commonly being in the vagina. Bacteremia is absent in most cases (Eykyn, 1982; Chesney, 1989). A penicillinase-resistant antibiotic, such as flucloxacillin, should be given parenterally to these patients, in addition to general supportive measures to combat shock. In some cases toxic shock syndrome has been associated with S. aureus septicemia and endocarditis (Whitby et al., 1983; Crowther and Ralph, 1993).
g. Cystic fibrosis
Respiratory colonization and infection with S. aureus is common in the early years in patients with cystic fibrosis. Prophylaxis with antistaphylococcal penicillins is commonly used in these patients. There are some short-term benefits in terms of reduced S. aureus infections, but the long-term benefits are unclear (Smyth and Walters, 2003).
References
Abrams B, Sklaver A, Hoffman T, Greenman R (1979). Single or combination
therapy of staphylococcal endocarditis in intravenous drug abusers. Ann
Intern Med 90: 789.
Ahmed ET, Mirghani OA, Gerais AS, Adam I (2004). Ceftriaxone versus
ampicillin/cloxacillin as antibiotic prophylaxis in elective caesarean section.
East Mediterr Health J 10: 277.
Amaya-Tapia G, Aguirre-Avalos G, Andrade-Villanueva J et al. (1993). Oncedaily
azithromycin in the treatment of adult skin and skin structure
infections. J Antimicrob Chemother 31 (Suppl E): 129.
Anderson P, Bluhm G, Herngren L, Jacobson B (1985). Pharmacokinetics and
distribution of flucloxacillin in pacemaker patients. Eur J Clin Pharmacol 27: 713.
Aronoff GR, Berns JS, Brier ME et al. (1999). In Drug prescribing in renal failure.
Dosing guidelines for adults (Aronoff GR et al., eds), 4th edn, Philadelphia,
PA: American College of Physicians.
Axline SG, Yaffe SJ, Simon HJ (1967). Clinical pharmacology of antimicrobials
in premature infants. II Ampicillin, methicillin, oxacillin, neomycin and
colistin. Pediatrics 39: 97.
Bakker SJ, Luik AJ, Leunissen KM (1995). Flucloxacillin-indbuced acute
interstitial nephritis. Nephrol Dial Transplant 10: 579.
Barber M, Waterworth PM (1964). Penicillinase-resistant penicillins and
cephalosporins. BMJ 2: 344.
Barton LL, Friedman AD, Portilla MG (1988). Inpetigo contagiosa: a
comparison of erythromycin and dicloxacillin therapy. Pediatr Dermatol 5: 88.
Barza M, Vine H, Weinstein L (1972). Reversibility of protein binding of
penicillins: an in vitro study employing a rapid diafiltration process.
Antimicrob Ag Chemother 1: 427.
See also
Lastest Price from Flucloxacillin manufacturers

US $0.00/kg2025-04-25
- CAS:
- 5250-39-5
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg