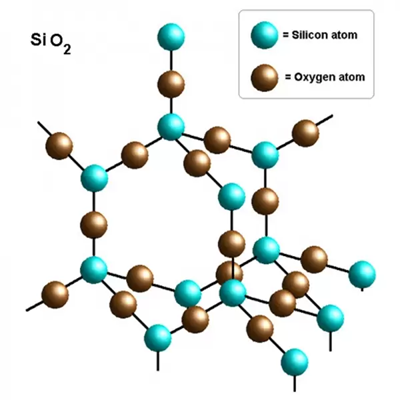
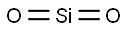Crystal Structure of Silicon dioxide
Silicon dioxide is a ubiquitous metal oxide widely used as a refractory, coating and nanocomposite material. Its silica nanoparticles are used in various fields such as catalysts, pigment stabilisation, modified concrete, electronics and sensors.
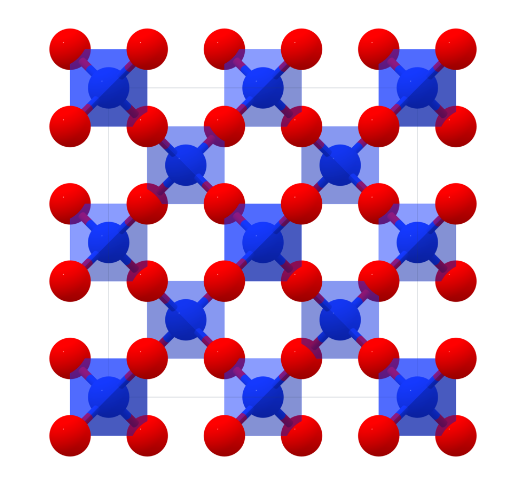
SiO2 is a polycrystalline compound, and all crystalline forms except wolframite and fibrous silica are tetrahedral SiO4 units joined together in different arrangements by sharing vertices. The length of the SiO4 bonds varies from crystal form to crystal form. E.g., quartz (α) structure of SiO₂, crystallising in the P3_221 tripartite space group.Si⁴⁺ bonds with four equivalent O² atoms to form the co-angled SiO₄ tetrahedron.The Si-O bond lengths have two shorter (1.61 Å) and two longer (1.62 Å) lengths.O² bonds in a curved 150-degree geometry to two equivalent Si⁴⁺ atoms.O² bonds with two equivalent Si⁴⁺ atoms.O² bonds with two equivalent O² atoms. - O² is bonded to two equivalent Si⁴⁺ atoms in a bent geometry using a 150 degree bend.
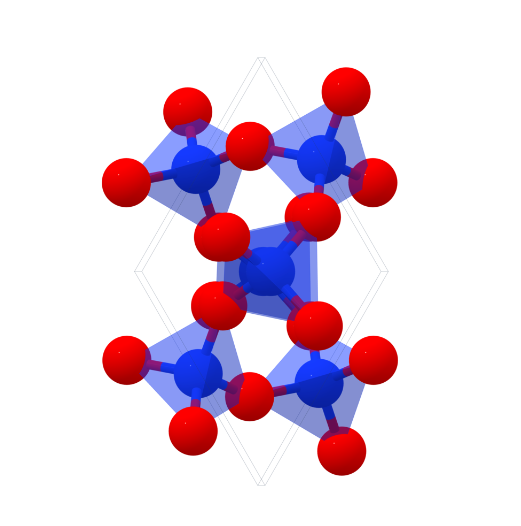
In addition, SiO₂ also has a high (β)-Christobalite structure, crystallising in the cubic Fd̅3m space group. Si⁴⁺ is bonded to four equivalent O² atoms to form the co-angled SiO₄ tetrahedra. O²- is bonded to two equivalent Si⁴ ⁺ atoms bonded in a linear geometry using.
Related articles And Qustion
See also
Lastest Price from Silicon dioxide manufacturers

US $150.00/kg2025-11-18
- CAS:
- 7631-86-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20tons

US $150.00/kg2025-11-18
- CAS:
- 7631-86-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20tons