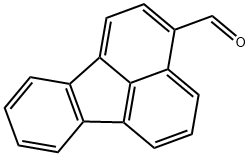
Fluoranthene-3-carbaldehyde: A Synthetic Intermediate
Fluoranthene-3-carbaldehyde is belong to the polycyclic aromatic hydrocarbon (PAH). The molecule can be viewed as the fusion of naphthalene and benzene unit connected by a five-membered ring. Although samples are often pale yellow, the compound is colorless. It is soluble in nonpolar organic solvents, but it is not as thermodynamically stable as pyrene. Its name is derived from its fluorescence under UV light [1].
Fluoranthene-3-carbaldehyde is always used as OLED intermediate in synthesis process.
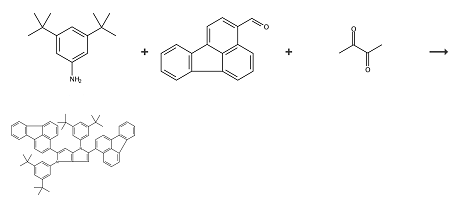
Scheme 1 The synthesis of 1,4-Bis(3,5-di-tert-butylphenyl)-2,5-bis(fluoranthen-3-yl)-1,4-di-hydropyrrolo[3,2-b]pyrrole
In the scheme 1, Fluoranthene-3-carbaldehyde acts as the material to synthesis the dye, 1,4-Bis(3,5-di-tert-butylphenyl)-2,5-bis(fluoranthen-3-yl)-1,4-di-hydropyrrolo[3,2-b]pyrrole. Fluoranthene-3-carbaldehyde (460.0 mg, 2 mmol), 3,5-di-tert-butylaniline (410.0 mg, 2 mmol), TsOH (34.4 mg, 20 mol-%) toluene (2 mL) and AcOH (2 mL) were placed in 50 mL two-necked round-bottomed flask equipped witha condenser and refluxed for 1 h. Subsequently, butane-2,3-dione(88 μL, 1 mmol) was added and the reaction was refluxed for an additional 3 h. After cooling, the crude product was precipitated byaddition of cold CH3CN, filtered off and recrystallized from hot EtOAc to afford 380.0 mg (42 %) of 1,4-Bis(3,5-di-tert-butylphenyl)-2,5-bis(fluoranthen-3-yl)-1,4-di-hydropyrrolo[3,2-b]pyrrole as an orange powder.
After photophysical property testing, more strikingly, redshift in absorption and emission spectra of pyrrolo[3,2-b]pyrrole bearing fluor-anthene groups is respectively 5500 cm–1 and 4300 cm–1 (almost 100 nm), which certainly denotes a marked change of the electronic properties. To get further insights into the electronic levels, the electro-chemical behavior of the synthesized compounds was investigated by means of cyclic voltammetry (CV). Tested compounds exhibited any well-developed reduction processes, however, it displayed first reversible oxidation wave around 0.58–0.74 V [2].
Scheme 2 shows the synthesis process of polycyclic fluoranthene derivatives of benz [a] anthracene. This approach involves the application of the ortho-lithiation methodology, which has recently been employed to prepare a broad range of polyaromatic compounds. 2-Lithio-iV- [2- (diethylamino)- ethyl] -IV-ethylbenzamide (1) was prepared by the method of Comins et al. and reacted with 3-formylfluoranthene to provide the lactone 3. Reduction of 3 with H2 over a Pd/C catalyst yielded the acid 4, which underwent cyclization with ZnCl2 in Ac20/AcOH to give 9-acetoxydibenz[e,fc]acephenanthrylene (5). Reduction of the lactone 30 with Zn in AcOH also provided 4, but this mode of reduction was very sensitive to the type of zinc used and gave an inferior yield. Treatment of 5 with Zn/NaOH gave dibenz[e,fe]acephenanthrylene (6) accompanied by a small amount of the quinone 7.
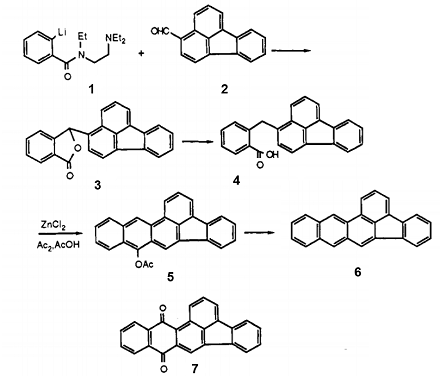
Scheme 2 Synthesis of polycyclic fluoranthene derivatives of benz [a] anthracene
References
[1] https://en.wikipedia.org/wiki/Fluoranthene.
[2] Banasiewicz, Marzena, Electronic Communication in Pyrrolo[3,2-b]pyrroles Possessing Sterically Hindered Aromatic Substituents, European Journal of Organic Chemistry, Volume2019, Issue31-32, 5247-5253
[3] Bongsup P. Cho and Ronald G. Harvey, Polycyclic Fluoranthene Hydrocarbons. 2. A New General Synthesis, J. Org. Chem. 1987, 52, 5668-5678
[4] http://www.chemspider.com/Chemical-Structure.157576.html?rid=80499bd8-814c-487b-b8c5-3fc282c71e12
[5] https://pubchem.ncbi.nlm.nih.gov/compound/181113