Synthesis, Detection and Bioactivity of Pazopanib Hydrochloride
Generally speaking
Pazopanib hydrochloride is a multi-targeted tyrosine kinase inhibitor that inhibits angiogenesis for the treatment of advanced renal cell carcinoma (RCC). Tyrosine kinase is the most common growth factor receptor, most RCC patients due to VHL gene mutation and abnormal growth of tumor tissue cause hypoxia-inducible factor (HIF)-related transcriptional activation, vascular endothelial growth factor (VEGF), platelet-derived Growth factor (PDGF), epidermal growth factor (EGF), transforming growth factor 2α (TGF2α) are overexpressed, and the Raf/MEK/ERK and PI3K/Akt/mTOR pathways are activated by autophosphorylation of receptor tyrosine kinases , make cells divide, proliferate and transform uncontrollably, stimulate new blood vessels, and promote tumor growth and metastasis. Pazopanib can selectively inhibit VEGFR-1, VEGFR-2, VEGFR-3, PDGFR-α, PDGFR-β and C-2Kit, and by blocking tyrosine kinases, it can destroy the signal transmission of tumor cells, thereby inhibiting the Tumor cell proliferation and angiogenesis, so as to achieve the purpose of anti-tumor.
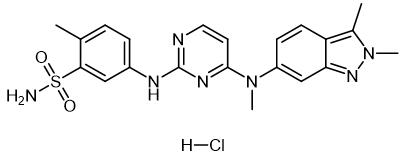
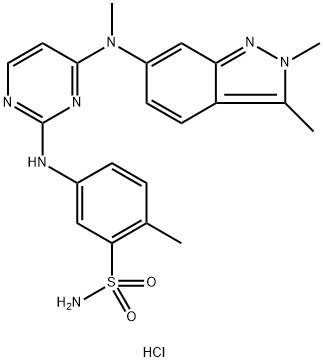
Fig. 1 The structure of Pazopanib Hydrochloride.
Synthetic routes
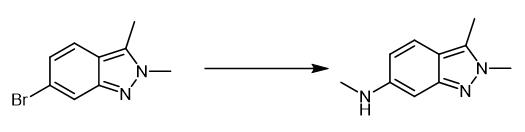
Fig. 2 The synthetic step 1 of Pazopanib Hydrochloride.
A flask was charged with 6-bromo-2,3-dimethy1 -2H-indazo1e (1 eq.), methylamine (2 eq.), Cu2O (5 mol%) and Ν,Ν-dimethylformamide. The reaction mixture was stirred at 100 ℃ until completion of the reaction. Then, the reaction mixture was cooled to room temperature and water was added. The mixture was extracted with dichloromethane. Organic layer was separated and dried with anhydrous sodium sulfate. The solvent was removed in vacuo to obtain oily residue. Diethyl ether was added onto the oily residue and stirred for 2 h for crystallization. Product crystals were collected by filtration, washed with diethyl ether and dried to afford a brownish powder (Yield: 97%; LC-purity: 99%) [1].
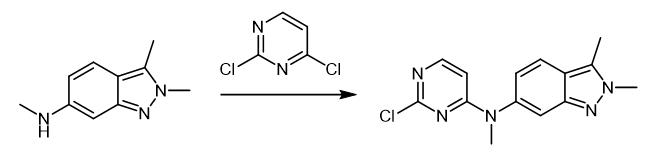
Fig. 3 The synthetic step 2 of Pazopanib Hydrochloride.
A flask was charged with N,2,3-trimethyl-2H-indazol-6-amine (1 eq.), 2,4-dichloropyrimidine (1.5 eq.), sodium bicarbonate (2 eq.) and Ν,Ν-dimethylformamide. The reaction mixture was stirred at 85 ℃ until completion of the reaction. Then, water was added and stirred for 3 h. Product crystals were collected by filtration, washed with water and then dried to get title compound as off-white/beige powder (Yield: 97%; LC-purity: 98.5%) [1].
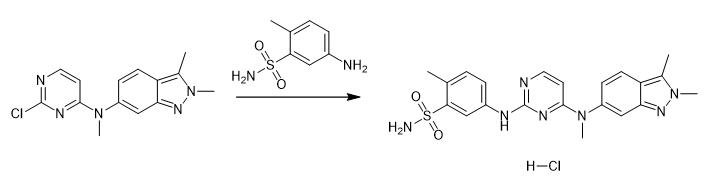
Fig. 4 The synthetic step 3 of Pazopanib Hydrochloride.
A flask was charged with intermediate synthesized in example 2 (N-(2-chloropyrimidi n-4-y1)-N,2,3-trimethyl-2H-indazol-6-amine) (1eq.), 5-amino-2-methylbenzenesulfonamide (1.05 eq.) and ethanol. Concentrated hydrochloric acid (3-4 drops) was added and the reaction mixture heated and stirred at reflux temperature until completion of the reaction which was monitored by thin layer chromatography (TLC). After completion of the reaction, product crystals were collected by filtration, washed with ethanol and dried to afford pazopanib hydrochloride as an off-white powder (Yield: 85%; LC-purity: 98.4%) [1].
Detection Method
A simple, accurate, precise, sensitive and stability indicating RP-HPLC method has been developed for the determination of Pazopanib hydrochloride in bulk drug and pharmaceutical dosage form, in which separations are done using develosil C18, 5 mu m, 150 x 4.6mm i.d. column at a flow rate of 1.0mL/min with an injection volume of 20 mu L. The beer's law was obeyed over the concentration range of 5 - 35 mu g/mL. The correlation coefficient was found to be 0.996 and it showed good linearity, reproducibility, precision in this concentration range. The aim of this paper was to develop and validate the stability indicating RP-HPLC method for the determination of Pazopanib hydrochloride in bulk and pharmaceutical dosage forms. The % recovery values were found to be within the limits, which showed that the method was accurate. The LOD and LOQ were calculated using statistical methods. The % RSD values were less than 2. The developed method was successfully applied for determination of Pazopanib hydrochloride in pharmaceutical dosage form. The results obtained are in good agreement with those obtained by using the standard method [2].
Understanding the origin and fate of organic impurities within the manufacturing process along with a good control strategy is an integral part of the quality control of drug substance. Following the underlying principles of quality by design (QbD), a systematic approach to analytical control of process impurities by impurity fate mapping (IFM) has been developed and applied to the investigation and control of impurities in the manufacturing process of Pazopanib hydrochloride, an anticancer drug approved recently by the U.S. FDA. This approach requires an aggressive chemical and analytical search for potential impurities in the starting materials, intermediates and drug substance, and experimental studies to track their fate through the manufacturing process in order to understand the process capability for rejecting such impurities. Comprehensive IFM can provide elements of control strategies for impurities. This paper highlights the critical roles that analytical sciences play in the IFM process and impurity control. The application of various analytical techniques (HPLC, LC-MS, NMR, etc.) and development of sensitive and selective methods for impurity detection, identification, separation and quantification are highlighted with illustrative examples. As an essential part of the entire control strategy for Pazopanib hydrochloride, analytical control of impurities with 'meaningful' specifications and the 'right analytical methods is addressed. In particular, IFM provides scientific justification that can allow for control of process impurities up-stream at the starting materials or intermediates whenever possible [3].
Thermal study
The currently marketed formulation of pazopanib hydrochloride has a poor bioavailability and pharmacokinetic profile. An alternative formulation of the drug might have an improved performance. Knowledge about the thermal behavior of the drug may be instrumental in the development process of such a formulation. The aim of this study was to thermally characterize raw bulk drug material of the multiple kinase inhibitor pazopanib hydrochloride using different analytical techniques. Solid-state characterization was carried out with Fourier transform infrared spectroscopy and X-ray diffraction. Thermal characterization was performed with thermogravimetric analysis and differential scanning calorimetry. Thermal and thermodynamic parameters were assessed using the Ozawa-Flynn-Wall, Friedman and Kissinger-Akahira-Sunose isoconversional methods [4].
Bioactivity
Suppression and Regression of Choroidal Neovascularization
To investigate pazopanib hydrochloride, a multitargeted kinase inhibitor, for treatment of choroidal neovascularization (CNV). Choroidal neovascularization was induced in mice by rupture of Bruch membrane with laser photocoagulation. Mice w
You may like
Related articles And Qustion
Lastest Price from Pazopanib Hydrochloride manufacturers

US $0.00/g2025-09-10
- CAS:
- 635702-64-6
- Min. Order:
- 1g
- Purity:
- More Than 99%
- Supply Ability:
- 50kg/Month

US $0.00/g2025-01-13
- CAS:
- 635702-64-6
- Min. Order:
- 1g
- Purity:
- More Than 99%
- Supply Ability:
- 100kg/Month