Synthesis and Application of 4,4'-(4,4'-Isopropylidenediphenyl-1,1'-diyldioxy)dianiline
Generally speaking
4,4'-(4,4'-Isopropylidenediphenyl-1,1'-diyldioxy)dianiline is a new type of heat-resistant bisamine curing agent, which is used in new polyester materials. Its melting point and boiling point are 127-130 °C and 587.1±50.0 °C, respectively. It is soluble in acetone and DMSO and very slightly soluble in water.
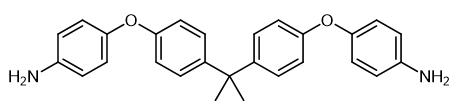
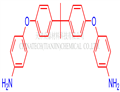
Fig. 1 The structure of 4,4'-(4,4'-Isopropylidenediphenyl-1,1'-diyldioxy)dianiline.
Synthetic routes

Fig. 2 The synthetic method 1 of 4,4'-(4,4'-Isopropylidenediphenyl-1,1'-diyldioxy)dianiline.
Disperse 2.5 g (5.3 mmol) 2,2-bis[4-(4-nitrophenoxy)phenyl] propane and 0.1 g of 10% Pd/C in 18 ml of ethanol and 2 ml tetrahydrofuran in a round-bottom flask, with magnetic stirring. Reflux a suspension solution and add about 5 ml of hydrazine hydrate to the mixture dropwise. Reflux the resultant reaction mixture at 85°C for 12.5 hours. Filter the hot black-colored solution to remove palladized charcoal. Pour the filtrate into a large amount of ice-cold water with efficient stirring. Filter a white crystal precipitate of 2,2-bis[4-(4-aminophenoxy)phenyl] propane, wash with ice-cold water. Dry the mixture at room temperature to obtain 2,2-bis[4-(4-aminophenoxy)phenyl] propane. 1H NMR (DMSO-d6, δ in ppm): 1.57 (s, 6H, CH3), 4.90 (s, 4H, NH2), 6.56-6.58 (m, 4H, Ar-H), 6.72-6.75 (m, 8H, ArH), 7.10-7.13 (3, 4H, Ar-H). IR FT (KBr, cm-1): 3489-3238 (NH2), 3040 (H-C aromatic), 2964 and 2869 (H-C aliphatic), 1612 (N-H), 1224 (C-O-C), 829 (parasubst. arom.). Elemental Analysis: Anal. calcd for C27H26N2O2 :C, 79.00%, H, 6.38%, N, 6.82%, Found: C, 78.17%, H, 6.59%, N, 6.35%. MP 130-132°C [1].

Fig. 3 The synthetic method 2 of 4,4'-(4,4'-Isopropylidenediphenyl-1,1'-diyldioxy)dianiline.
2,2-Bis[4-(4-nitrophenoxy)phenyl]propane (3; 5.0 g, 10.6 mmol) dissolved in ethyl acetate (100 mL) and palladium on activated carbon (0.30 g) was placed in a hydrogenation bottle. The bottle was tightly secured on a Parr hydrogenation apparatus, flushed four times with hydrogen, and pressurized to 55 psi. After the mixture had been agitated at room temperature for 24 h under the hydrogen pressure of 55 psi, it was filtered through Celite. The filter cake was washed with ethyl acetate, and then the filtrate was evaporated to dryness on a rotary evaporator. Off-white crystals, yield 4.214 g, 95%. mp 126.5-127.5°C. IR (KBr, cm-1): 3423, 3402, 3333, 3235 (amine), 3038, 2964, 2869,1879, 1733, 1610, 1498, 1222 (ether), 872. 1H NMR(DMSO-d6, δ in ppm): 1.55 (s, 6H, CH3), 4.94 (s, 4H, NH2), 6.55-6.71(m, 4H, Ar-H), 6.71- 6.77 (m, 8H, Ar-H), 7.11-7.13 (m, 4H, Ar-H). Anal. Calcd for C27H26N2O2 :C,79.00%,H,6.38%,N,6.82%,Found:C,78.27%,H, 6.65%, N, 6.45% [2].

Fig. 3 The synthetic method 3 of 4,4'-(4,4'-Isopropylidenediphenyl-1,1'-diyldioxy)dianiline.
Put ether (0.1 mol), activated carbon (5.0 g), ferric chloride (1.0 g) and ethylene glycol monomethyl ether (150 ml) in a flask. Heat the reaction mixture at 110°C. Stir the reaction mixture for half an hour. Add hydrazine (85%, 0.5 mole) to the reaction mixture. Reflux the reaction mixture for 8 hours. To prevent the product from separating out, hot filter the reaction mixture. Wash the reaction mixture with ethylene glycol monomethyl ether. Neutralize the filtrate with hydrochloric acid (20%, 300 ml). When white precipitates appears, add ammonia to the reaction mixture to neutralize the excessive hydrochloric acid until a pH of 11-12 is achieved. Wash the precipitate with distilled water. Crystallize the product from ethanol and a 1,4-dioxane mixture [3].
Application
Effects on the Structure, Curing Mechanism and Properties of Polyacrylate Elastomers
To develop high-performance polyacrylicester (ACM) elastomeric components with higher scorch safety and superior thermal and mechanical properties, we replaced aliphatic diamine curatives with aromatic dianiline curatives. The influence of dianiline curatives 4,4'-(4,4'-isopropylidenediphenyl-1,1'-diyldioxy)dianiline, 4,4'-(hexafluoroisopropylidene)bis(p-phenyleneoxy)dianiline, and 4,4'-(1,1'-biphenyl-4,4'-diyldioxy)dianiline on the network structures and thermal, dynamic mechanical, and mechanical properties of ACM vulcanizates was investigated. The kinetics of vulcanization was analyzed for different dianiline curatives, with the use of rheometer curves. To understand the electronic properties and study the relation between chemical structure and reactivity, density functional theory was used. The time-temperature superposition principal was used to evaluate the activation energy for degradation of cross-linked samples. Finally, the curing mechanism of ACM in the presence of dianiline curative was studied with X-ray photoelectron spectroscopy and Fourier transform infrared spectroscopy. These spectroscopic analyses suggested that the reaction mechanism took place via two steps: the first step was formation of the amide linkage and the second step was formation of imide linkages [4].
Physical and thermal properties of ethylene terephthalate fabrics
Two analogous polyimides (PIs) containing flexible isopropylidene units were prepared. One was based on 4,4-oxydiphthalic anhydride and 4,4-(4,4-isopropylidenediphenyl-1,1-diyldioxy) dianiline and the other was based on 4,4-(4,4-isopropylidenediphenoxy) bis(phthalic anhydride) and bis(3-aminophenyl) methyl phosphine oxide. The ability of these two PIs to form uniform nanoscaled fibers was investigated by electrospinning technique. At optimal spinning conditions, PI fibers were electrospun onto the surface of woven poly(ethylene terephthalate) (PET) support to form a bilayer composite structure. These new fabric systems were analyzed regarding morphology, air permeability, wetting properties, and thermal stability. It was expected that the new PET/PI mats would possess enhanced properties compared with the initial woven PET fibers due to the excellent properties of PIs. Experimental results showed that PET woven substrate coated with electrospun PI fibers had improved values of air permeability, water contact angle and thermal stability when compared with the initial woven PET fibers [5].
Form carbon membranes
Polyimide membranes were synthesized by the polymerization of five diamines, p-phenylenediamine (PPD), 2,3,5,6-tetramethyl-1,4-phenylenediamine (TMPPD), 4,4'-oxydianiline (ODA), 4,4'-(4,4'-isopropylidenediphenyl-1,1'-diyldioxy) dianiline (BAPP) and 4,4'-(hexafluoroisopropylidene) bis(p-phenyleneoxy) dianiline (BDAF), and the same dianhydride monomer, 1,2,4,5-benzenetetracarboxylic anhydride (PMDA). The chemical structures of the membranes pyrolyzed at different temperatures were investigated by TGA, TG-MS, FT-IR and XPS. Results indicate that there are five stages during pyrolysis (a) the removal of the solvents, adsorbed oxygen and water, (b) imidiz
Related articles And Qustion
Lastest Price from 2,2-Bis[4-(4-aminophenoxy)phenyl]propane manufacturers
US $0.00-0.00/Kg2025-10-27
- CAS:
- 13080-86-9
- Min. Order:
- 1Kg
- Purity:
- ≥99.9%
- Supply Ability:
- 50MT

US $0.00/KG2025-04-21
- CAS:
- 13080-86-9
- Min. Order:
- 1KG
- Purity:
- 98%min
- Supply Ability:
- 30tons/month