Synthesis and Biological Activity of Trilostane
Generally speaking
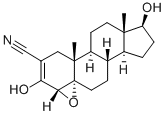
Trilostane, namely 4α, 5α-epoxy-2-cyanoandrostere-2-ene-3, 17β-diol, can reversibly inhibit 3β-hydroxysteroid dehydrogenase and △5, 4-isomase in adrenal cortex, thereby blocking the biosynthesis of salt corticosteroids and glucocorticoids. In 1980, trilostane was approved in the United Kingdom to treat aldosteronism and hypercortisol. It was approved in the United States in 1985 to treat Cushing's syndrome, or hyperadrenocortical hyperactivity. The drug is also approved for veterinary use in the UK because older animals, particularly dogs, are prone to Cushing's syndrome, and trilostane relieves symptoms in more than 90% of dogs and improves their quality of life. Trilostane itself has no hormonal activity, so its side effects are few, and its safety and tolerability are particularly good. Breast cancer is a hormone-dependent tumor, and estrogen is the main driver of the growth of this cancer cell. Therefore, one of the major modern therapies for breast cancer is to target estrogen, either by inhibiting estrogen production or by blocking the action of estrogen at its site of action, the estrogen receptor link. The estrogen receptor was originally thought to be a single receptor, but recent studies have identified at least two alpha and beta subtypes. Among them, estrogen can stimulate cell growth when it binds to α-estrogen receptors, but when it binds to β-estrogen receptors, it downregulates α-estrogen receptors and slows down the rate of cell proliferation. Studies have revealed that in addition to reducing estrogen production, Trilostane can also regulate the binding of estrogen to different subtypes of estrogen receptors, showing both alpha-estrogen receptor inhibitors and beta-estrogen receptor agonists. The dual effects of Chemicalbook ultimately block and alter the negative effects of Chemicalbook estrogen on cancer cells. Trilostam's unique mode of action not only sets it apart from other existing antiestrogens, but is also the pharmacological basis for its continued high efficacy in breast cancers that have failed or are resistant to other antiestrogens.
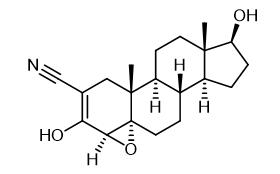
Fig. 1 The structure of Trilostane.
Synthetic routes
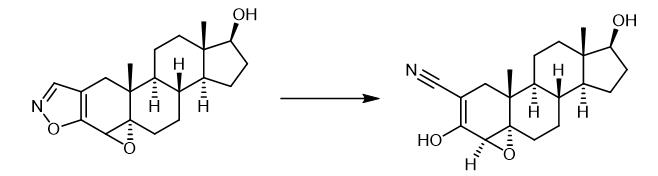
Fig. 2 The synthetic of Trilostane.
The following procedure may be used for the conversion of (4α,5α,17β)-4,5-epoxyandrost-2-eno(2,3-d)isoxazol-17-ol (1) to Trilostane ((4α,5α,17β)-4,5-epoxy-3,17-dihydroxyandrost-2-ene-2-carbonitrile (2). Process 1. Charge compound (1) (10.0 g, 30.4 mmols, 1.0 equiv) to a round-bottomed flask fitted with a stirrer. 2. Charge methanol (150 ml) and sodium hydroxide (1.49 g, 37.3 mmols, 1.23 equivs) to the flask and leave to stir-out. 3. Leave the solution to stir-out with warming (40 - 45 °C, two hours). 4. Hold the temperature of the stirred reaction mixture (40 - 45 °C) and slowly charge acetic acid (2.74 g, 45.6 mmols, 1.50 equiv) over two-hours. 5. Add water (150 ml) in minimum time consistent with maintaining the temperature of the resulting slurry at 40 - 45°C. 6. Leave the slurry to stir-out and cool to 18 - 20 °C. 7. Collect the solid by filtration and wash with water. 8. Charge filter cake to a vacuum oven to dry (at 40 - 50°C) to constant weight. The above procedure provided. Trilostane (2) of 9.5 g (95%). [1].
Bioactivity
Hyperadrenocorticism
Background: Dogs treated for naturally occurring hyperadrenocorticism (NOH) in Korea often appear to require higher doses of trilostane than recommended by authors in the United States, Europe, or the United Kingdom. This phenomenon may be related to compounding trilostane into packets, which is a common practice among veterinary clinics in Korea. Objective: Analyze packets filled by hand and others filled using a semi-automatic packing device for accuracy of trilostane strength. Animals Medication packets prepared for 3 dogs with preexisting prescriptions for NOH were analyzed. Method: A trilostane assay was developed for analysis. Trilostane (Vetoryl) capsules were used as clinical controls. Forty-four medication packets containing trilostane (Vetoryl), prepared by 3 clinicians for 3 dogs with NOH were analyzed. Results: Of 44 trilostane-containing packets, only 40.9% (18 packets) had acceptable strength of trilostane. Conclusions and Clinical Importance: Clinicians should be aware that compounding trilostane into packets fails to consistently provide measured amounts of trilostane, potentially interfering with response to treatment for NOH in dogs [2].
Inflammation and nociception
BACKGROUND: Trilostane was identified in an in vivo screen of compounds in a lipopolysaccharide model of inflammation to support a repurposing effort. There is no previous documentation of any anti-inflammatory effects of trilostane. OBJECTIVE: The aim of this study was to elucidate the novel pharmacologic activity of trilostane in a series of inflammation and nociception signal-finding models. METHODS: Anti-inflammatory effects of trilostane were evaluated in lipopolysaccharide-induced systemic and lung inflammation models and in a 2,4-dinitrofluorobenzene-induced delayed-type hypersensitivity (DTH) model in the mouse ear. The analgesic activities of trilostane were evaluated in a hot plate nociception model as a function of paw-withdrawal latency and in the formalin-induced nociception model with a behavioral end point. In all studies, trilostane was administered 15 minutes before challenge. In the DTH model, the animals were given a second dose 24 hours after the first dose. RESULTS: Trilostane inhibited tumor necrosis factor-alpha and monocyte chemoattractant protein-1 production in the lipopolysaccharide-induced systemic and pulmonary inflammation models. It also significantly reduced ear swelling in the 2,4-dinitrofluorobenzene-induced DTH model. In the hot plate nociception model, trilostane increased the latency of paw-licking behavior. Trilostane also significantly reduced the duration of pain behaviors in the late phase of the formalin-induced inflammatory pain model. CONCLUSIONS: These signal-finding studies suggest that trilostane has novel anti-inflammatory and analgesic properties [3].
Hypercortisolism
Background: Glucocorticoids influence the synthesis and metabolism of catecholamines (epinephrine and norepinephrine) and metanephrines (metanephrine and normetanephrine). The aim of this study was to measure urinary catecholamines and metanephrines in dogs with hypercortisolism before and during trilostane therapy. Urine samples were collected during initial work up and during therapy with trilostane in 14 dogs with hypercortisolism and in 25 healthy dogs. Epinephrine, norepinephrine, metanephrine and normetanephrine were measured using high-pressure liquid chromatography and expressed as ratios to urinary creatinine concentration. Results: Untreated dogs with hypercortisolism had significantly higher epinephrine, norepinephrine, and normetanephrine: creatinine ratios compared to healthy dogs. During trilostane therapy, urinary catecholamines and their metabolites did not decrease significantly. However, dogs with low post-ACTH cortisol concentrations during trilostane therapy had less increased epinephrine, norepinephrine and normetanephrine: creatinine ratios compared to healthy dogs. There was no correlation of urinary catecholamines and their metabolites with baseline or post-ACTH cortisol or endogenous ACTH concentrations during trilostane therapy. Conclusion:
Related articles And Qustion
See also
Lastest Price from Trilostane manufacturers

US $0.00/KG2025-09-01
- CAS:
- 13647-35-3
- Min. Order:
- 1KG
- Purity:
- 0.99
- Supply Ability:
- 1TON

US $1.00/KG2025-07-02
- CAS:
- 13647-35-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10000